Label: CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE tablet
- NDC Code(s): 62559-660-90, 62559-661-90, 62559-662-90
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 14, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
- •
- When pregnancy is detected, discontinue candesartan cilexetil and hydrochlorothiazide tablets as soon as possible.
- •
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See Warnings: Fetal Toxicity
-
DESCRIPTION
Candesartan Cilexetil and Hydrochlorothiazide Tablets USP combine an angiotensin II receptor (type AT1) antagonist and a diuretic, hydrochlorothiazide.
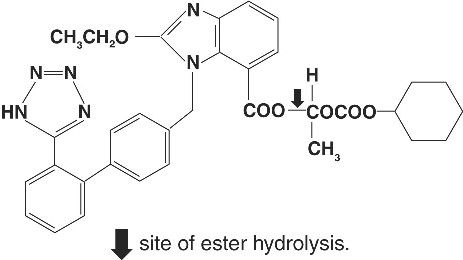
Candesartan cilexetil, a nonpeptide, is chemically described as (±)-1-Hydroxyethyl 2-ethoxy-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-7-benzimidazolecarboxylate, cyclohexyl carbonate (ester).
Its empirical formula is C33H34N6O6 and its structural formula is:
Candesartan cilexetil is a white to off-white powder with a molecular weight of 610.67. It is practically insoluble in water and sparingly soluble in methanol. Candesartan cilexetil is a racemic mixture containing one chiral center at the cyclohexyloxycarbonyloxy ethyl ester group. Following oral administration, candesartan cilexetil undergoes hydrolysis at the ester link to form the active drug, candesartan, which is achiral.
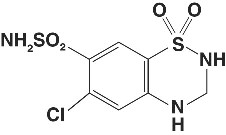
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.72, which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
Candesartan cilexetil and hydrochlorothiazide tablets are available for oral administration in three tablet strengths of candesartan cilexetil and hydrochlorothiazide.
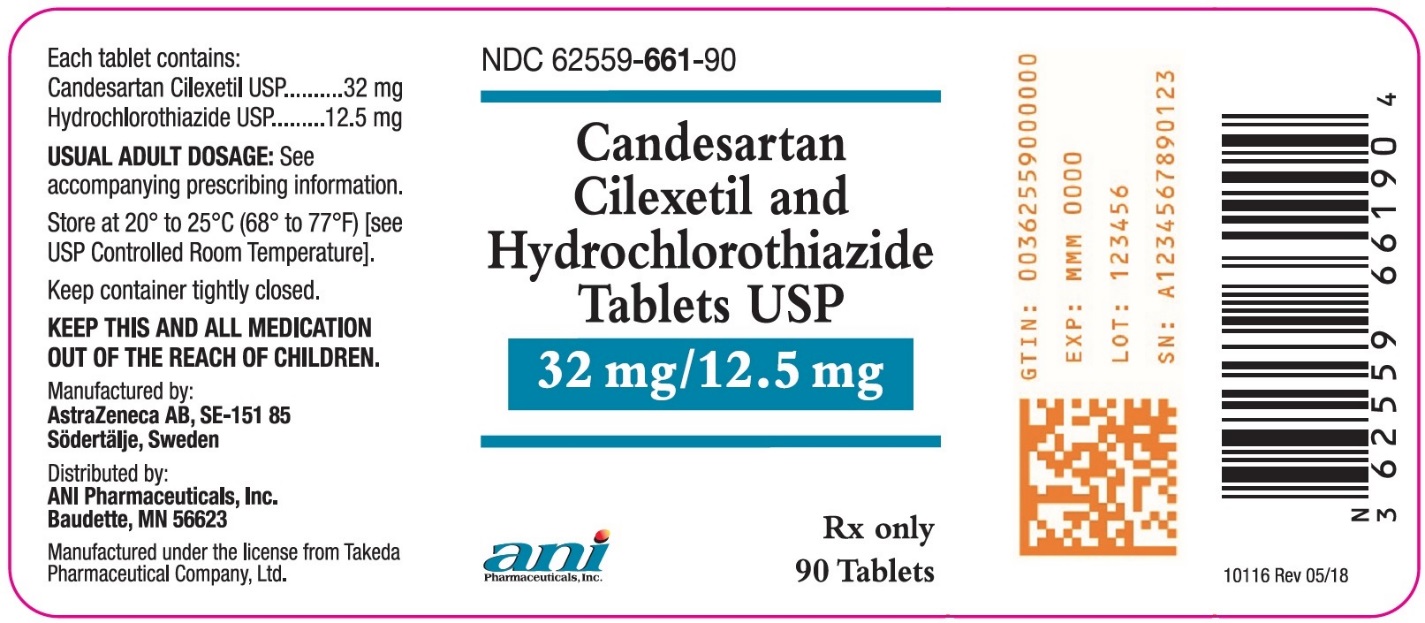
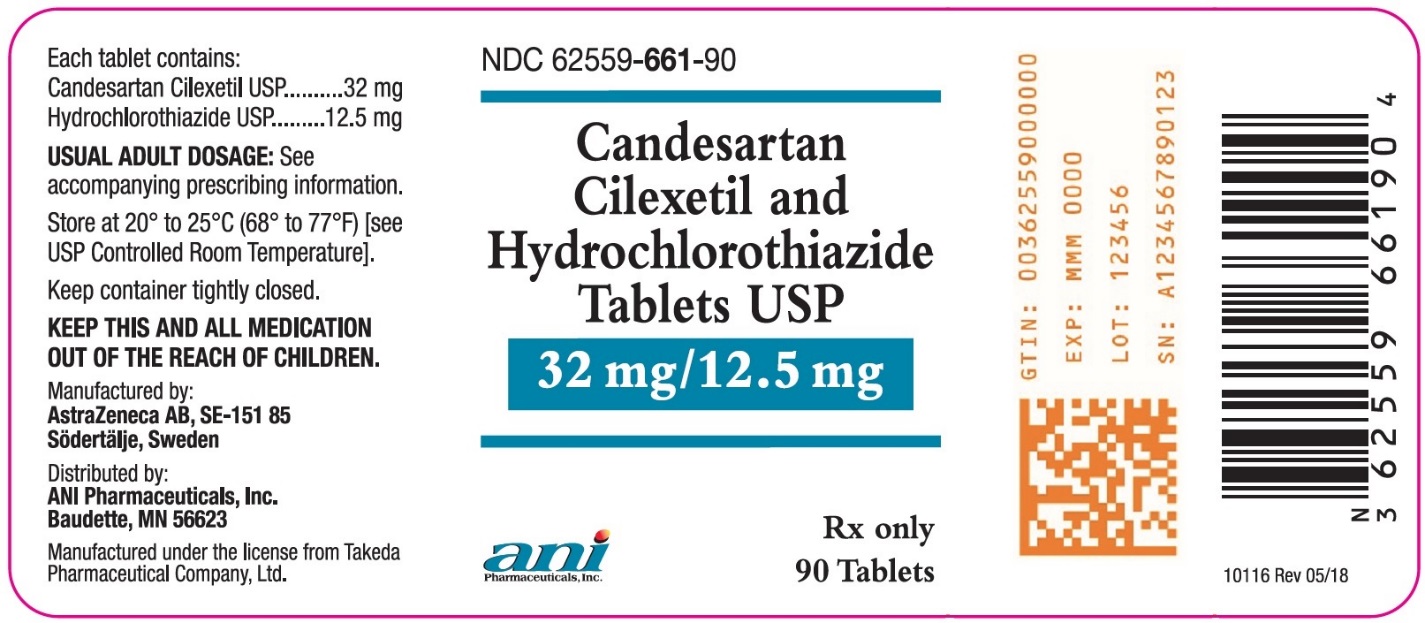
Candesartan cilexetil and hydrochlorothiazide tablets contain 16 mg or 32 mg of candesartan cilexetil and 12.5 mg or 25 mg of hydrochlorothiazide providing for the following available combinations: 16 mg/12.5 mg, 32 mg/12.5 mg or 32 mg/25 mg. The inactive ingredients of the tablets are carboxymethylcellulose calcium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, corn starch, polyethylene glycol 8000, and ferric oxide (yellow). Ferric oxide (reddish brown) is also added to the 16 mg/12.5 mg and 32 mg/25 mg tablets as colorant.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Candesartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is, therefore, independent of the pathways for angiotensin II synthesis.
There is also an AT2 receptor found in many tissues, but AT2 is not known to be associated with cardiovascular homeostasis. Candesartan has much greater affinity (>10,000-fold) for the AT1 receptor than for the AT2 receptor.
Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because candesartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Candesartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of candesartan on blood pressure.
Hydrochlorothiazide is a thiazide diuretic. Thiazides affect the renal tubular mechanisms of electrolyte reabsorption, directly increasing excretion of sodium and chloride in approximately equivalent amounts. Indirectly, the diuretic action of hydrochlorothiazide reduces plasma volume, with consequent increases in plasma renin activity, increases in aldosterone secretion, increases in urinary potassium loss, and decreases in serum potassium. The renin-aldosterone link is mediated by angiotensin II, so co‑administration of an angiotensin II receptor antagonist tends to reverse the potassium loss associated with these diuretics.
The mechanism of the antihypertensive effect of thiazides is unknown.
Pharmacokinetics
General
Candesartan Cilexetil
Candesartan cilexetil is rapidly and completely bioactivated by ester hydrolysis during absorption from the gastrointestinal tract to candesartan, a selective AT1 subtype angiotensin II receptor antagonist. Candesartan is mainly excreted unchanged in urine and feces (via bile). It undergoes minor hepatic metabolism by O-deethylation to an inactive metabolite. The elimination half-life of candesartan is approximately 9 hours. After single and repeated administration, the pharmacokinetics of candesartan are linear for oral doses up to 32 mg of candesartan cilexetil. Candesartan and its inactive metabolite do not accumulate in serum upon repeated once-daily dosing.
Following administration of candesartan cilexetil, the absolute bioavailability of candesartan was estimated to be 15%. After tablet ingestion, the peak serum concentration (Cmax) is reached after 3 to 4 hours. Food with a high fat content does not affect the bioavailability of candesartan after candesartan cilexetil administration.
Hydrochlorothiazide
When plasma levels have been followed for at least 24 hours, the plasma half-life has been observed to vary between 5.6 and 14.8 hours.
Metabolism and Excretion
Candesartan Cilexetil
Total plasma clearance of candesartan is 0.37 mL/min/kg, with a renal clearance of 0.19 mL/min/kg. When candesartan is administered orally, about 26% of the dose is excreted unchanged in urine. Following an oral dose of 14C-labeled candesartan cilexetil, approximately 33% of radioactivity is recovered in urine and approximately 67% in feces. Following an intravenous dose of 14C-labeled candesartan, approximately 59% of radioactivity is recovered in urine and approximately 36% in feces. Biliary excretion contributes to the elimination of candesartan.
Hydrochlorothiazide
Hydrochlorothiazide is not metabolized but is eliminated rapidly by the kidney. At least 61% of the oral dose is eliminated unchanged within 24 hours.
Distribution
Candesartan Cilexetil
The volume of distribution of candesartan is 0.13 L/kg. Candesartan is highly bound to plasma proteins (>99%) and does not penetrate red blood cells. The protein binding is constant at candesartan plasma concentrations well above the range achieved with recommended doses. In rats, it has been demonstrated that candesartan crosses the blood-brain barrier poorly, if at all. It has also been demonstrated in rats that candesartan passes across the placental barrier and is distributed in the fetus.
Hydrochlorothiazide
Hydrochlorothiazide crosses the placental but not the blood-brain barrier and is excreted in breast milk.
Special Populations
Pediatric
The pharmacokinetics of candesartan cilexetil have not been investigated in patients <18 years of age.
Geriatric
The pharmacokinetics of candesartan have been studied in the elderly (≥ 65 years). The plasma concentration of candesartan was higher in the elderly (Cmax was approximately 50% higher, and AUC was approximately 80% higher) compared to younger subjects administered the same dose. The pharmacokinetics of candesartan were linear in the elderly, and candesartan and its inactive metabolite did not accumulate in the serum of these subjects upon repeated, once-daily administration. No initial dosage adjustment is necessary (see DOSAGE AND ADMINISTRATION).
Gender
There is no difference in the pharmacokinetics of candesartan between male and female subjects.
Renal Insufficiency
In hypertensive patients with renal insufficiency, serum concentrations of candesartan were elevated. After repeated dosing, the AUC and Cmax were approximately doubled in patients with severe renal impairment (creatinine clearance <30 mL/min/1.73m2) compared to patients with normal kidney function. The pharmacokinetics of candesartan in hypertensive patients undergoing hemodialysis are similar to those in hypertensive patients with severe renal impairment. Candesartan cannot be removed by hemodialysis.
Thiazide diuretics are eliminated by the kidney, with a terminal half-life of 5-15 hours. In a study of patients with impaired renal function (mean creatinine clearance of 19 mL/min), the half-life of hydrochlorothiazide elimination was lengthened to 21 hours (see DOSAGE AND ADMINISTRATION).
Safety and effectiveness of candesartan cilexetil and hydrochlorothiazide tablets in patients with severe renal impairment (CrCL ≤30 mL/min) have not been established. No dose adjustment is required in patients with mild (CrCL 60-90 mL/min) or moderate (CrCL 30-60 mL/min) renal impairment.
Hepatic Insufficiency
The pharmacokinetics of candesartan were compared in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment to matched healthy volunteers following a single dose of 16 mg candesartan cilexetil. The AUC for candesartan in patients with mild and moderate hepatic impairment was increased 30% and 145% respectively. The Cmax for candesartan was increased 56% and 73% respectively. The pharmacokinetics of candesartan in severe hepatic impairment have not been studied. No dose adjustment is recommended for patients with mild hepatic impairment. In patients with moderate hepatic impairment, candesartan cilexetil and hydrochlorothiazide tablets are not recommended for initiation because the appropriate starting dose, 8 mg, cannot be given (see DOSAGE AND ADMINISTRATION).
Monitor patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
Pharmacodynamics
Candesartan Cilexetil
Candesartan inhibits the pressor effects of angiotensin II infusion in a dose-dependent manner. After 1 week of once-daily dosing with 8 mg of candesartan cilexetil, the pressor effect was inhibited by approximately 90% at peak with approximately 50% inhibition persisting for 24 hours.
Plasma concentrations of angiotensin I and angiotensin II, and plasma renin activity (PRA), increased in a dose-dependent manner after single and repeated administration of candesartan cilexetil to healthy subjects and hypertensive patients. ACE activity was not altered in healthy subjects after repeated candesartan cilexetil administration. The once-daily administration of up to 16 mg of candesartan cilexetil to healthy subjects did not influence plasma aldosterone concentrations, but a decrease in the plasma concentration of aldosterone was observed when 32 mg of candesartan cilexetil was administered to hypertensive patients. In spite of the effect of candesartan cilexetil on aldosterone secretion, very little effect on serum potassium was observed.
In multiple-dose studies with hypertensive patients, there were no clinically significant changes in metabolic function including serum levels of total cholesterol, triglycerides, glucose, or uric acid. In a 12-week study of 161 patients with non-insulin-dependent (type 2) diabetes mellitus and hypertension, there was no change in the level of HbA1c.
Hydrochlorothiazide
After oral administration of hydrochlorothiazide, diuresis begins within 2 hours, peaks in about 4 hours and lasts about 6 to 12 hours.
Clinical Trials
Candesartan Cilexetil and Hydrochlorothiazide
Of 12 controlled clinical trials involving 4588 patients, 5 were double-blind, placebo controlled and evaluated the antihypertensive effects of single entities vs the combination. These 5 trials, of 8 to 12 weeks duration, randomized 3037 hypertensive patients. Doses ranged from 2 to 32 mg candesartan cilexetil and from 6.25 to 25 mg hydrochlorothiazide administered once daily in various combinations.
The combination of candesartan cilexetil and hydrochlorothiazide resulted in placebo-adjusted decreases in sitting systolic and diastolic blood pressures of 14-18/8-11 mm Hg at doses of 16 mg/12.5 mg and 32 mg/12.5 mg. The combination of candesartan cilexetil and hydrochlorothiazide 32 mg/25 mg resulted in placebo-adjusted decreases in sitting systolic and diastolic blood pressures of 16-19/9-11 mm Hg. The placebo corrected trough to peak ratio was evaluated in a study of candesartan cilexetil and hydrochlorothiazide 32 mg/12.5 mg and was 88%.
Most of the antihypertensive effect of the combination of candesartan cilexetil and hydrochlorothiazide was seen in 1 to 2 weeks with the full effect observed within 4 weeks. In long-term studies of up to 1 year, the blood pressure lowering effect of the combination was maintained. The antihypertensive effect was similar regardless of age or gender, and overall response to the combination was similar in black and non-black patients. No appreciable changes in heart rate were observed with combination therapy in controlled trials.
-
INDICATIONS AND USAGE
Candesartan cilexetil and hydrochlorothiazide tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with candesartan cilexetil and hydrochlorothiazide tablets.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
This fixed dose combination is not indicated for initial therapy (see DOSAGE AND ADMINISTRATION).
-
CONTRAINDICATIONS
Candesartan cilexetil and hydrochlorothiazide tablets are contraindicated in patients who are hypersensitive to candesartan, to hydrochlorothiazide or to other sulfonamide-derived drugs.
Do not co-administer aliskiren with candesartan cilexetil and hydrochlorothiazide tablets in patients with diabetes (see PRECAUTIONS, Drug Interactions).
Candesartan cilexetil and hydrochlorothiazide tablets are contraindicated in patients with anuria.
-
WARNINGS
Fetal Toxicity
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue candesartan cilexetil and hydrochlorothiazide tablets as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue candesartan cilexetil and hydrochlorothiazide tablets, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to candesartan cilexetil and hydrochlorothiazide tablets for hypotension, oliguria, and hyperkalemia (see PRECAUTIONS, Pediatric Use).
There was no evidence of teratogenicity or other adverse effects on embryo-fetal development when pregnant mice, rats or rabbits were treated orally with candesartan cilexetil alone or in combination with hydrochlorothiazide. For mice, the maximum dose of candesartan cilexetil was 1000 mg/kg/day (about 150 times the maximum recommended daily human dose [MRHD]1). For rats, the maximum dose of candesartan cilexetil was 100 mg/kg/day (about 31 times the MRHD1). For rabbits, the maximum dose of candesartan cilexetil was 1 mg/kg/day (a maternally toxic dose that is about half the MRHD1). In each of these studies, hydrochlorothiazide was tested at the same dose level (10 mg/kg/day, about 4, 8, and 15 times the MRHD1 in mouse, rats, and rabbit, respectively). There was no evidence of harm to the rat or mouse fetus or embryo in studies in which hydrochlorothiazide was administered alone to the pregnant rat or mouse at doses of up to 1000 and 3000 mg/kg/day, respectively.
Thiazides cross the placental barrier and appear in cord blood. There is a risk of fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
- 1
- Doses compared on the basis of body surface area. MRHD considered to be 32 mg for candesartan cilexetil and 12.5 mg for hydrochlorothiazide.
Hypotension
Candesartan cilexetil and hydrochlorothiazide tablets can cause symptomatic hypotension. Symptomatic hypotension is most likely to occur in patients who have been volume and/or salt depleted as a result of prolonged diuretic therapy, dietary salt restriction, dialysis, diarrhea, or vomiting. Patients with symptomatic hypotension may require temporarily reducing the dose of candesartan cilexetil and hydrochlorothiazide tablets or volume repletion. Volume and/or salt depletion should be corrected before initiating therapy with candesartan cilexetil and hydrochlorothiazide tablets.
In patients with heart failure, candesartan cilexetil and hydrochlorothiazide tablets may cause excessive hypotension, which may lead to oliguria, azotemia, and (rarely) with acute renal failure and death (see WARNINGS, Impaired Renal Function). In such patients, candesartan cilexetil and hydrochlorothiazide tablets therapy should be started under close medical supervision; they should be followed closely for the first 2 weeks of treatment and whenever the dose of candesartan or diuretic is increased.
Impaired Renal Function
Monitor renal function periodically in patients treated with candesartan cilexetil and hydrochlorothiazide tablets. Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system and by diuretics. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe heart failure, or volume depletion) may be at particular risk of developing oliguria, progressive azotemia, or acute renal failure on candesartan cilexetil and hydrochlorothiazide tablets. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on candesartan cilexetil and hydrochlorothiazide tablets.
Potassium Abnormalities
Drugs that inhibit the renin-angiotensin system can cause hyperkalemia. Hydrochlorothiazide can cause hypokalemia and hyponatremia. Hypomagnesemia can result in hypokalemia, which appears difficult to treat despite potassium repletion. Monitor serum electrolytes periodically.
In clinical trials of various doses of candesartan cilexetil and hydrochlorothiazide, the incidence of hypertensive patients who developed hypokalemia (serum potassium <3.5 mEq/L) was 2.5% versus 2.1% for placebo; the incidence of hyperkalemia (serum potassium >5.7 mEq/L) was 0.4% versus 1.0% for placebo. No patient receiving candesartan cilexetil and hydrochlorothiazide tablets 16 mg/12.5 mg or 32 mg/12.5 mg was discontinued due to increases or decreases in serum potassium.
Acute Myopia and Secondary Angle-Closure Glaucoma
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
-
PRECAUTIONS
Metabolic Disturbances
Hydrochlorothiazide may alter glucose tolerance and raise serum levels of cholesterol and triglycerides.
Hydrochlorothiazide may raise the serum uric acid level due to reduced clearance of uric acid and may cause or exacerbate hyperuricemia and precipitate gout in susceptible patients.
Thiazides decrease urinary calcium excretion and may cause elevation of serum calcium. Avoid using candesartan cilexetil and hydrochlorothiazide tablets in patients with hypercalcemia.
Systemic Lupus Erythematosus
Thiazide diuretics have been reported to cause exacerbation or activation of systemic lupus erythematosus.
Information for Patients
Pregnancy
Female patients of childbearing age should be told about the consequences of exposure to candesartan cilexetil and hydrochlorothiazide tablets during pregnancy. Discuss treatment options with women planning to become pregnant. Patients should be asked to report pregnancies to their physicians as soon as possible.
Symptomatic Hypotension
Tell patients receiving candesartan cilexetil and hydrochlorothiazide tablets that lightheadedness can occur, especially during the first days of therapy, and that it should be reported to the prescribing physician. Tell patients that if syncope occurs, discontinue candesartan cilexetil and hydrochlorothiazide tablets until the physician has been consulted.
Tell all patients that inadequate fluid intake, excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope.
Hyperkalemia
Tell patients receiving candesartan cilexetil and hydrochlorothiazide tablets not to use potassium supplements, salt substitutes containing potassium, or other drugs that may increase serum potassium levels without consulting the prescribing physician.
Non-melanoma Skin Cancer
Instruct patients taking hydrochlorothiazide to protect skin from the sun and undergo regular skin cancer screening.
Drug Interactions
Because candesartan is not significantly metabolized by the cytochrome P450 system and at therapeutic concentrations has no effects on P450 enzymes, interactions with drugs that inhibit or are metabolized by those enzymes would not be expected.
Interactions common to both Candesartan Cilexetil and Hydrochlorothiazide
Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists, including candesartan, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving candesartan and NSAID therapy.
The antihypertensive effect of angiotensin II receptor antagonists, including candesartan may be attenuated by NSAIDs including selective COX-2 inhibitors.
Lithium
Increases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II receptor antagonists or hydrochlorothiazide. Monitor serum lithium levels during concomitant use.
Interactions with Candesartan Cilexetil
Dual Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Closely monitor blood pressure, renal function and electrolytes in patients on candesartan cilexetil and hydrochlorothiazide tablets and other agents that affect the RAS.
Co-administration of candesartan cilexetil and hydrochlorothiazide tablets with potassium sparing diuretics, potassium supplements, potassium-containing salt substitutes or other drugs that raise serum potassium levels may result in hyperkalemia. Monitor serum potassium in such patients.
Do not co-administer aliskiren with candesartan cilexetil and hydrochlorothiazide tablets in patients with diabetes. Avoid use of aliskiren with candesartan cilexetil and hydrochlorothiazide tablets in patients with renal impairment (GFR <60 mL/min) (see CONTRAINDICATIONS).
Interactions with Hydrochlorothiazide
Alcohol, barbiturates, or narcotics − Potentiation of orthostatic hypotension may occur.
Antidiabetic drugs (oral agents and insulin) − Dosage adjustment of the antidiabetic drug may be required.
Diazoxide − the hyperglycemic effect of diazoxide may be enhanced by thiazides.Ion Exchange resins − Single doses of either cholestyramine or colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85% and 43%, respectively. Stagger the dosage of hydrochlorothiazide and ion exchange resins such that hydrochlorothiazide is administered at least 4 hours before or 4-6 hours after the administration of resins.
Skeletal muscle relaxants, nondepolarizing (e.g., tubocurarine) − Possible increased responsiveness to muscle relaxants such as curare derivatives.
Digitalis − Thiazide-induced hypokalemia or hypomagnesemia may predispose to digoxin toxicity.
Noradrenaline – Thiazides may decrease arterial responsiveness to noradrenaline, but not enough to preclude effectiveness of the pressor agent for therapeutic use.Steroids or Adrenocorticotropic Hormone – Hypokalemia may develop during concomitant use of steroids or adrenocorticotropic hormone (ACTH).
Cytotoxic products – Thiazides may reduce the renal excretion of cytotoxic medicinal products (e.g. cyclophosphamide, methotrexate) and potentiate their myelosuppressive effects.
Cyclosporine − Concomitant treatment with cyclosporine may increase the risk of hyperuricemia and gout-type complications.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been conducted with the combination of candesartan cilexetil and hydrochlorothiazide. There was no evidence of carcinogenicity when candesartan cilexetil was orally administered to mice and rats for up to 104 weeks at doses up to 100 and 1000 mg/kg/day, respectively. Rats received the drug by gavage whereas mice received the drug by dietary administration. These (maximally-tolerated) doses of candesartan cilexetil provided systemic exposures to candesartan (AUCs) that were, in mice, approximately 7 times and, in rats, more than 70 times the exposure in man at the maximum recommended daily human dose (32 mg). Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice (at doses of up to approximately 600 mg/kg/day) or in male and female rats (at doses of up to approximately 100 mg/kg/day). The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Candesartan cilexetil or candesartan (the active metabolite), in combination with hydrochlorothiazide, tested positive in vitro in the Chinese hamster lung (CHL) chromosomal aberration assay and mouse lymphoma mutagenicity assay. The candesartan cilexetil/hydrochlorothiazide combination tested negative for mutagenicity in bacteria (Ames test), for unscheduled DNA synthesis in rat liver, for chromosomal aberrations in rat bone marrow and for micronuclei in mouse bone marrow.
Both candesartan and its O-deethyl metabolite tested positive for genotoxicity in the in vitro CHL chromosomal aberration assay. Neither compound tested positive in the Ames microbial mutagenesis assay or in the in vitro mouse lymphoma cell assay. Candesartan (but not its O-deethyl metabolite) was also evaluated in vivo in the mouse micronucleus test and in vitro in the Chinese hamster ovary (CHO) gene mutation assay, in both cases with negative results. Candesartan cilexetil was evaluated in the Ames test, the in vitro mouse lymphoma cell assay, the in vivo rat hepatocyte unscheduled DNA synthesis assay and the in vivo mouse micronucleus test, in each case with negative results. Candesartan cilexetil was not evaluated in the CHL chromosomal aberration or CHO gene mutation assays.
When hydrochlorothiazide was tested alone, positive results were obtained in vitro in the CHO sister chromatid exchange (clastogenicity) and mouse lymphoma cell (mutagenicity) assays and in the Aspergillus nidulans non-disjunction assay. Hydrochlorothiazide was not genotoxic in vitro in the Ames test for point mutations and the CHO test for chromosomal aberrations, or in vivo in assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene.
No fertility studies have been conducted with the combination of candesartan cilexetil and hydrochlorothiazide. Fertility and reproductive performance were not affected in studies with male and female rats given oral doses of up to 300 mg candesartan cilexetil/kg/day (83 times the maximum daily human dose of 32 mg on a body surface area basis). Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to conception and throughout gestation.
Nursing Mothers
It is not known whether candesartan is excreted in human milk, but candesartan has been shown to be present in rat milk. Thiazides appear in human milk. Because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Neonates with a history of in utero exposure to candesartan cilexetil and hydrochlorothiazide tablets:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
Safety and effectiveness in pediatric patients have not been established.
-
ADVERSE REACTIONS
Candesartan Cilexetil and Hydrochlorothiazide
Candesartan cilexetil and hydrochlorothiazide tablets have been evaluated for safety in more than 2800 patients treated for hypertension. More than 750 of these patients were studied for at least six months and more than 500 patients were treated for at least one year. Adverse experiences have generally been mild and transient in nature and have only infrequently required discontinuation of therapy. The overall incidence of adverse events reported with candesartan cilexetil and hydrochlorothiazide tablets was comparable to placebo. The overall frequency of adverse experiences was not related to dose, age, gender, or race.
In placebo-controlled trials that included 1089 patients treated with various combinations of candesartan cilexetil (doses of 2-32 mg) and hydrochlorothiazide (doses of 6.25-25 mg) and 592 patients treated with placebo, adverse events, whether or not attributed to treatment, occurring in greater than 2% of patients treated with candesartan cilexetil and hydrochlorothiazide tablets and that were more frequent for candesartan cilexetil and hydrochlorothiazide tablets than placebo were: Respiratory System Disorder: upper respiratory tract infection (3.6% vs 3.0%); Body as a Whole: back pain (3.3% vs 2.4%); influenza-like symptoms (2.5% vs 1.9%); Central/Peripheral Nervous System: dizziness (2.9% vs 1.2%).
Post-Marketing Experience
The following have been very rarely reported in post-marketing experience with candesartan cilexetil:
Digestive: Abnormal hepatic function and hepatitis.
Hematologic: Neutropenia, leukopenia, and agranulocytosis.
Immunologic: Angioedema
Metabolic and Nutritional Disorders: Hyperkalemia, hyponatremia.
Respiratory System Disorders: Cough
Skin and Appendages Disorders: Pruritus, rash and urticaria.
Rare reports of rhabdomyolysis have been reported in patients receiving angiotensin II receptor blockers.
Hydrochlorothiazide
Other adverse experiences that have been reported with hydrochlorothiazide, without regard to causality, are listed below:
Gastrointestinal: pancreatitis, jaundice (intrahepatic cholestatic jaundice), sialadenitis, cramping, constipation, gastric irritation, anorexia
Hematologic: aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia
Hypersensitivity: anaphylactic reactions, necrotizing angiitis (vasculitis and cutaneous vasculitis), respiratory distress including pneumonitis and pulmonary edema, photosensitivity, urticaria, purpura
Musculoskeletal: muscle spasm
Non-melanoma Skin Cancer: Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥50,000 mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year.
Skin: erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis, alopecia
Special Senses: transient blurred vision, xanthopsia
Urogenital: impotence
-
OVERDOSAGE
Candesartan Cilexetil and Hydrochlorothiazide
No lethality was observed in acute toxicity studies in mice, rats and dogs given single oral doses of up to 2000 mg/kg of candesartan cilexetil or in rats given single oral doses of up to 2000 mg/kg of candesartan cilexetil in combination with 1000 mg/kg of hydrochlorothiazide. In mice given single oral doses of the primary metabolite, candesartan, the minimum lethal dose was greater than 1000 mg/kg but less than 2000 mg/kg.
Limited data are available in regard to overdosage with candesartan cilexetil in humans. The most likely manifestations of overdosage with candesartan cilexetil would be hypotension, dizziness, and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation. If symptomatic hypotension should occur, supportive treatment should be initiated. For hydrochlorothiazide, the most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
Candesartan cannot be removed by hemodialysis. The degree to which hydrochlorothiazide is removed by hemodialysis has not been established.
Treatment
To obtain up-to-date information about the treatment of overdose, consult your Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians’ Desk Reference (PDR). In managing overdose, consider the possibilities of multiple-drug overdoses, drug-drug interactions, and altered pharmacokinetics in your patient.
-
DOSAGE AND ADMINISTRATION
The usual recommended starting dose of candesartan cilexetil is 16 mg once daily when it is used as monotherapy in patients who are not volume depleted. Candesartan cilexetil tablets can be administered once or twice daily with total daily doses ranging from 8 mg to 32 mg. Patients requiring further reduction in blood pressure should be titrated to 32 mg. Doses larger than 32 mg do not appear to have a greater blood pressure lowering effect.
Hydrochlorothiazide is effective in doses of 12.5 to 50 mg once daily.
Use in Renal Impairment: Dosing recommendations for candesartan cilexetil and hydrochlorothiazide tablets in patients with creatinine clearance < 30 mg/min cannot be provided (see SPECIAL POPULATIONS, Renal Insufficiency).
Use in moderate to severe Hepatic Impairment: Candesartan cilexetil and hydrochlorothiazide tablets are not recommended for initiation because the appropriate starting dose, 8 mg, cannot be given (see SPECIAL POPULATIONS, Hepatic Insufficiency).
Replacement Therapy: The combination may be substituted for the titrated components.
Dose Titration by Clinical Effect: A patient whose blood pressure is not controlled on 25 mg of hydrochlorothiazide once daily can expect an incremental effect from candesartan cilexetil and hydrochlorothiazide tablets 16 mg/12.5 mg. A patient whose blood pressure is controlled on 25 mg of hydrochlorothiazide but is experiencing decreases in serum potassium can expect the same or incremental blood pressure effects from candesartan cilexetil and hydrochlorothiazide tablets 16 mg/12.5 mg and serum potassium may improve.
A patient whose blood pressure is not controlled on 32 mg of candesartan cilexetil tablets can expect incremental blood pressure effects from candesartan cilexetil and hydrochlorothiazide tablets 32 mg/12.5 mg and then 32 mg/25 mg. The maximal antihypertensive effect of any dose of candesartan cilexetil and hydrochlorothiazide tablets can be expected within 4 weeks of initiating that dose.
Candesartan cilexetil and hydrochlorothiazide tablets may be administered with other antihypertensive agents.
Candesartan cilexetil and hydrochlorothiazide tablets may be administered with or without food.
-
HOW SUPPLIED
Candesartan Cilexetil and Hydrochlorothiazide Tablets USP, 16 mg/12.5 mg are peach, oval, biconvex, non-film-coated tablets, scored on both sides and coded with ACS on one side. They are supplied in bottles of 90 tablets (NDC 62559-660-90).
Candesartan Cilexetil and Hydrochlorothiazide Tablets USP, 32 mg/12.5 mg are yellow, oval, biconvex, non-film-coated tablets, scored on both sides and coded with ACJ on one side. They are supplied in bottles of 90 tablets (NDC 62559-661-90).
Candesartan Cilexetil and Hydrochlorothiazide Tablets USP, 32 mg/25 mg are pink, oval, biconvex, non-film-coated tablets, scored on both sides and coded with ACD on one side. They are supplied in bottles of 90 tablets (NDC 62559-662-90).
Storage:
Store at 20° to 25°C (68° to 77°F); excursions permitted at 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep container tightly closed.
Manufactured under the license from Takeda Pharmaceutical Company, Ltd.
Manufactured by:
AstraZeneca AB, SE-151 85
Södertälje, SwedenDistributed by:
ANI Pharmaceuticals, Inc.
Baudette, MN 56623

10118 Rev 05/20
- PRINCIPAL DISPLAY PANEL – 16 mg/12.5 mg
- PRINCIPAL DISPLAY PANEL – 32 mg/12.5 mg
- PRINCIPAL DISPLAY PANEL – 32 mg/25 mg
-
INGREDIENTS AND APPEARANCE
CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
candesartan cilexetil and hydrochlorothiazide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62559-660 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDESARTAN CILEXETIL (UNII: R85M2X0D68) (CANDESARTAN - UNII:S8Q36MD2XX) CANDESARTAN CILEXETIL 16 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color PINK (peach) Score 2 pieces Shape OVAL (biconvex) Size 10mm Flavor Imprint Code ACS Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62559-660-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA021093 09/26/2018 CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
candesartan cilexetil and hydrochlorothiazide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62559-661 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDESARTAN CILEXETIL (UNII: R85M2X0D68) (CANDESARTAN - UNII:S8Q36MD2XX) CANDESARTAN CILEXETIL 32 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 12.5 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW Score 2 pieces Shape OVAL (biconvex) Size 11mm Flavor Imprint Code ACJ Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62559-661-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA021093 09/26/2018 CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
candesartan cilexetil and hydrochlorothiazide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62559-662 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDESARTAN CILEXETIL (UNII: R85M2X0D68) (CANDESARTAN - UNII:S8Q36MD2XX) CANDESARTAN CILEXETIL 32 mg HYDROCHLOROTHIAZIDE (UNII: 0J48LPH2TH) (HYDROCHLOROTHIAZIDE - UNII:0J48LPH2TH) HYDROCHLOROTHIAZIDE 25 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color PINK Score 2 pieces Shape OVAL (biconvex) Size 11mm Flavor Imprint Code ACD Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62559-662-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA021093 09/26/2018 Labeler - ANI Pharmaceuticals, Inc. (145588013)