Label: ERWINASE- asparaginase injection, powder, lyophilized, for solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 81561-413-01, 81561-413-05 - Packager: Porton Biopharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 15, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HEALTH CARE PROVIDER LETTER
25 May 2021
IMPORTANT PRESCRIBING INFORMATION
Subject: Temporary importation of Erwinase® (crisantaspase) injection, powder, lyophilized, for solution to address a drug shortage in the United States (U.S.)
Dear Healthcare Professional,
In order to alleviate a critical shortage of U.S.-licensed asparaginase erwinia chrysanthemi injection, powder, lyophilized, for solution in the US market, Porton Biopharma Limited (PBL) is coordinating with the U.S. Food and Drug Administration (FDA) to make available in the U.S. the non-FDA licensed Erwinase (crisantaspase) 10,000 IU/vial powder for solution for injection/infusion.
At this time, no other entity except PBL (via its distributor Clinigen Inc.) is authorized by the FDA to import and distribute Erwinase in the U.S. However, this does not represent a formal FDA approval of Erwinase in the U.S.
Effective immediately, PBL (via its distributor Clinigen Inc.) will distribute the following presentation of Erwinase (Batch number: W060172) to address the critical shortage:
Product Name
Pack Size
Description
U.K. Marketing Authorization Number
NDC
Erwinase, 10,000 IU/vial, Powder for solution for injection/infusion.
5 vials
Powder for solution for injection/infusion. White lyophilised powder in a vial.
PL 44403/0002
NDC 81561-413-05*
*This NDC code has been assigned by PBL. The code is presented on the carton. It should be noted that the importer and distributor in the U.S. will be Clinigen Inc. The same NDC code will be used throughout the distribution chain of the product in the U.S.
It is important to note the following:
- Erwinase is available in the U.S. only by prescription.
-
There is no barcode on this product for use with U.S. barcode scanning systems.
Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients. -
Contraindications to use of Erwinase include:
- Serious hypersensitivity reactions to asparaginase erwinia chrysanthemi, including anaphylaxis
- Serious pancreatitis with any prior asparaginase therapy
- Serious thrombosis with any prior asparaginase therapy
- Serious hemorrhagic events with any prior asparaginase therapy
-
There are differences between the U.S.-licensed asparaginase erwinia chrysanthemi Prescribing Information and the Erwinase Summary of Product Characteristics (Appendix 2). Use the recommended dosage for U.S.-licensed asparaginase erwinia chrysanthemi
- To substitute for each planned dose of pegaspargase, the recommended dosage of Erwinase is 25,000 International Units/m2 administered intramuscularly or intravenously three times a week (Monday/Wednesday/Friday) for two consecutive weeks (total six doses).
- When administering Erwinase intravenously, the desired NSAA levels may not be achieved; consider monitoring nadir (pre-dose) serum asparaginase activity (NSAA) levels and switching to intramuscular administration if desired NSAA levels are not achieved with intravenous dosing.
-
There are differences between the U.S.-licensed product and Erwinase in the Preparation and Handling Instructions (Appendix 2). Use the recommended handling instruction for U.S.-licensed asparaginase erwinia chrysanthemi.
- Do not freeze or refrigerate reconstituted solution and administer within 4 hours or discard.
-
There are differences between the U.S.-licensed product and Erwinase in the format and content of the container and carton labelling.
- The Erwinase carton includes an insert providing both the U.K. prescribing information (Summary of Product Characteristics) and information for the patient (package leaflet). See the bullet above regarding the recommended dosage instructions for Erwinase.
- The vial label will display the text used and approved for marketing the product in U.K. Please see the product comparison tables at the end of this letter (Appendix 1).
- The packaging of Erwinase does not include serialization information and does not meet the product identifier requirements of section 582(b)(2) of the Federal Food, Drug and Cosmetic Act.
Ensure that your staff and others in your office and/or pharmacy who may be involved in the prescribing and/or dispensing of Erwinase- asparaginase injection, powder, lyophilized, for solution receive a copy of this letter, review the information and instruct patients on the differences between Erwinase and U.S.-licensed asparaginase erwinia chrysanthemi.
This letter and the attachments are not intended as a complete description of the benefits and risks related to the use of Erwinase. See the full prescribing information on the UK MHRA website at https://products.mhra.gov.uk/product/?product=ERWINASE%2010%20000%20UNITS%2FVIAL%20%20LYOPHILISATE%20FOR%20SOLUTION%20FOR%20INJECTION
Contact Information:
If you have any questions about the information contained in this letter, any quality related problems, or questions on the use of Erwinase, please contact Porton Biopharma Limited via email medinfo@portonbiopharma.com or contact directly at 1-855-209-2652.
To place an order, contact McKesson Plasma and Biologics (MPB) directly at 1-877-625-2566.
Adverse Events and Product Quality Complaints:
Report adverse events associated with the use of Erwinase to drugsafety@portonbiopharma.com via email or contact directly at 1-855-209-2652.
Adverse events, medication errors or quality problems experienced with the use of Erwinase may also be reported to the FDA’s MedWatch Adverse Event Reporting Program either online, regular mail, or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/medwatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
We remain at your disposal to answer any questions you may have about our product and to provide more information if needed.
Yours sincerely,
Dr. Elizabeth Madichie
Dr. Henno Welgemoed
Director of Regulatory Affairs & Pharmacovigilance
Director of Medical Affairs
Porton Biopharma Limited, U.K.
Clinigen Group plc, U.K.
Enclosures:
Appendix 1 – Product Label and Product Characteristics Side-by-Side Comparison Table
Appendix 2 – Prescribing Information Side-by-Side Comparison Table
Appendix 1 – Product Label and Product Characteristics Side-by-Side Comparison Table
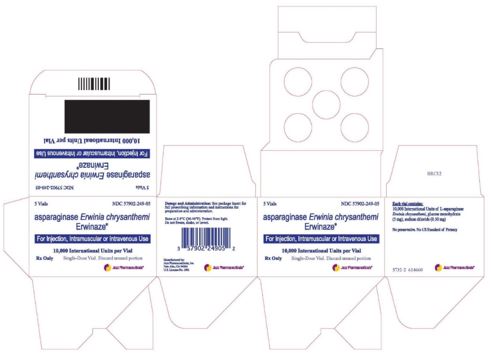
U.S. FDA Licensed Product
Imported Product
Product Name
Erwinaze®- asparaginase injection, powder, lyophilized, for solution
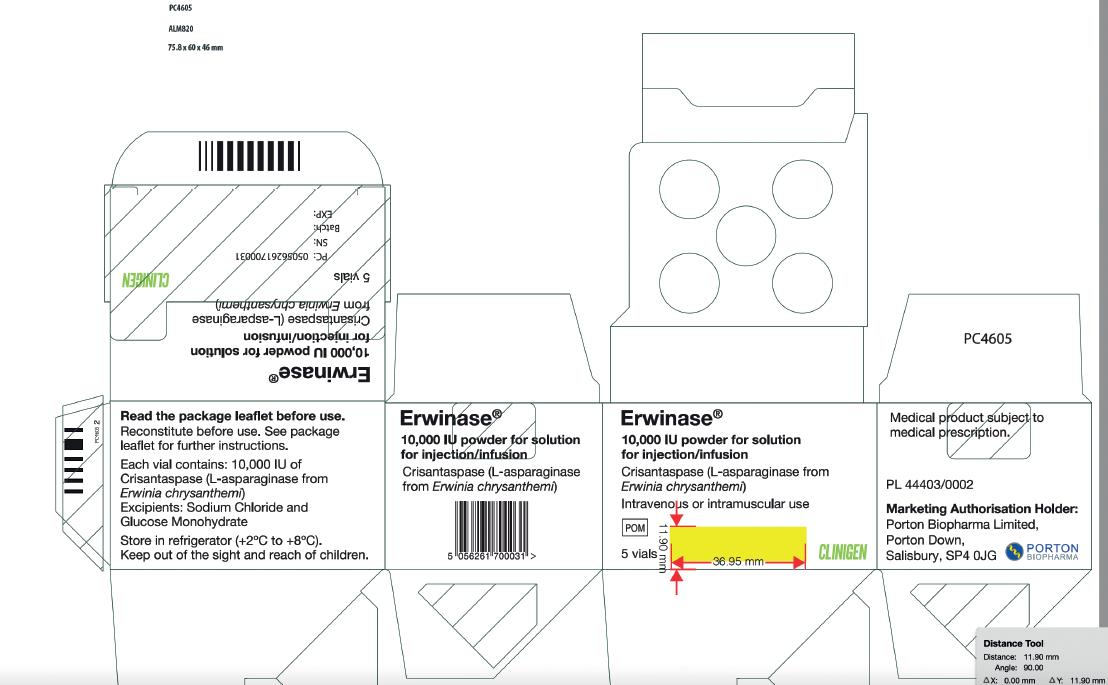
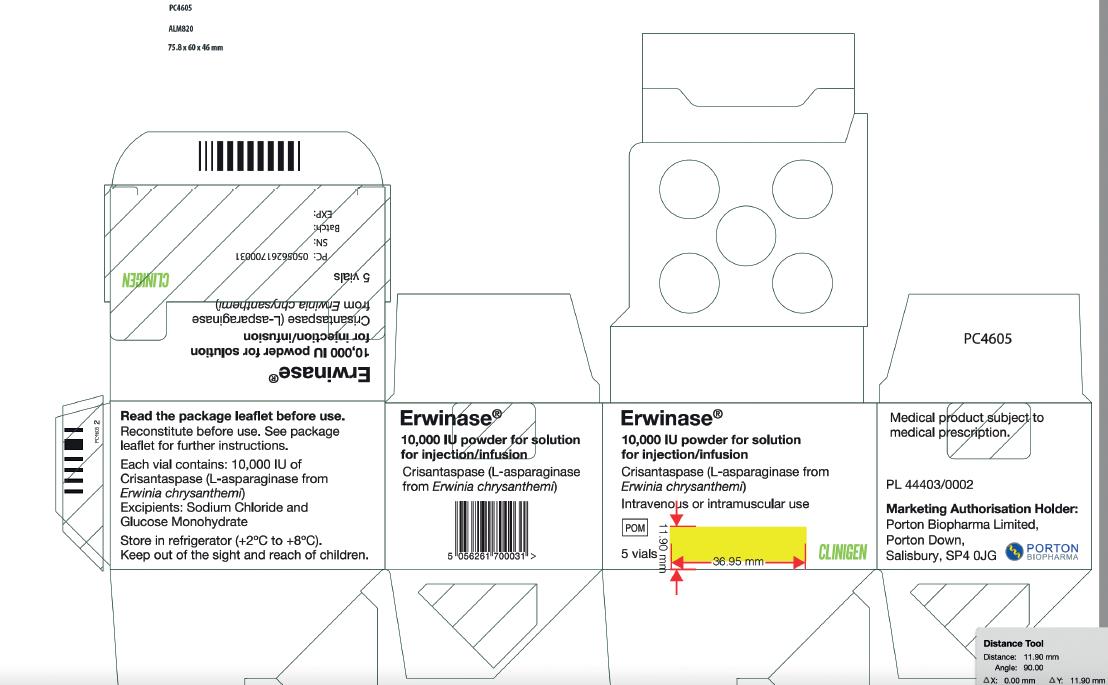
Erwinase® 10,000 IU/vial Powder for solution for injection/infusion
Strength
10,000 International Units in a single-dose vial
10,000 IU/vial
Carton Label Image
Vial Label Image


Route of Administration
Erwinaze can be administered intramuscularly or intravenously.
Erwinase solution can be given by intravenous infusion or intramuscular injection.
Ingredients
Erwinaze is supplied as a sterile, lyophilized, white powder in vials. Each vial contains 10,000 International Units of asparaginase Erwinia chrysanthemi, and the following inactive ingredients: glucose monohydrate (5.0 mg), sodium chloride (0.5 mg).
Crisantaspase (L-asparaginase from Erwinia chrysanthemi), 10,000 International units/vial.
List of excipients
Sodium Chloride
Glucose Monohydrate
Storage Conditions
Store unused or unopened vials and cartons at 36°F to 46°F (2°C to 8°C). Protect from light. Do not use Erwinaze after the expiration date on the vial.
Store in a refrigerator (+2°C to +8°C).
Appendix 2 – Prescribing Information Side-by-Side Comparison Table
A side-by-side comparison of the Erwinaze U.S. Prescribing Information (USPI) and Erwinase U.K. Summary of Product Characteristics (SmPC) is provided below. It is important to note that there are no significant differences in the indications, dosage and administration between the two products.
Side-by-Side Comparison Table:
U.S. FDA Licensed Product
Imported Product
Product name
Erwinaze®- asparaginase injection, powder, lyophilized, for solution
Erwinase® 10,000 IU/vial Powder for solution for injection/infusion
Indication
1 Indications and Usage
ERWINAZE is indicated as a component of a multi-agent chemotherapeutic regimen for the treatment of patients with acute lymphoblastic leukemia (ALL) who have developed hypersensitivity to E. coli derived asparaginase.
4 Clinical Particulars
4.1 Therapeutic indications
Erwinase is indicated as a component of a chemotherapeutic regimen for the treatment of patients with acute lymphoblastic leukaemia (ALL) who have developed hypersensitivity to E. coli-derived asparaginase.
Erwinase is indicated in paediatric patients from the age of 4 months and in adults.
Dosage and administration
2 Dosage and administration
2.1 Recommended Dosage
To substitute for a dose of pegaspargase:
The recommended dose for each planned dose of pegaspargase is 25,000 International Units/m2 administered intramuscularly or intravenously three times a week (Monday/Wednesday/Friday) for six doses.
To substitute for a dose of native E. coli asparaginase:
The recommended dose is 25,000 International Units/m2 administered intramuscularly or intravenously for each scheduled dose of native E. coli asparaginase within a treatment.
When administering ERWINAZE intravenously, consider monitoring nadir (pre-dose) serum asparaginase activity (NSAA) levels and switching to intramuscular administration if desired NSAA levels are not achieved [see Clinical Pharmacology (12.3)].
4.2 Posology and method of administration
Posology
The recommended dosage is 20,000 or 25,000 IU/m2 body surface area administered three times a week (e.g., Monday/Wednesday/Friday).
Therapy should be adjusted according to local treatment protocols.
2.2 Preparation and Handling Instructions
- Visually inspect the ERWINAZE powder for foreign particulate matter and discoloration prior to reconstitution. Discard vial if present.
- Reconstitute the contents of each vial by slowly injecting 1 or 2 mL of preservative free sterile sodium chloride (0.9%) injection (USP) against the inner vial wall.
- Do not forcefully inject solution for reconstitution directly onto or into the powder. When reconstituted with 1 mL the resultant concentration is 10,000 International Units per mL. When reconstituted with 2 mL the resultant concentration is 5,000 International Units per mL.
- Dissolve contents by gentle mixing or swirling. Do not shake or invert vial.
- When reconstituted, ERWINAZE should be a clear, colorless solution. Inspect the solution after reconstitution and discard if any visible particles or protein aggregates are present.
6.6 Special precautions for disposal and other handling
The contents of each vial should be reconstituted in 1 mL to 2 mL of sodium chloride (0.9%) solution for injection.
When reconstituted with 1 mL the resultant concentration is 10,000 IU/mL. When reconstituted with 2 mL the resultant concentration is 5,000 IU/mL
Slowly add the sodium chloride (0.9%) solution for injection. against the inner vial wall, do not squirt directly onto or into the powder.
Allow the contents to dissolve by gentle mixing or swirling maintaining the vial in an upright position, avoiding contact of the solution with the stopper. Avoid froth formation due to excessive or vigorous shaking.
The solution should be clear without any visible particles. Fine crystalline or thread-like wisps of protein aggregates may be visible if shaking is excessive. If there are any visible particles or protein aggregates present the reconstituted solution should be rejected.
- Calculate the dose needed and the volume needed to obtain the calculated dose.
- Withdraw the volume containing the calculated dose from the vial into a polypropylene syringe within 15 minutes of reconstitution. For intravenous use, slowly inject the reconstituted ERWINAZE into an IV infusion bag containing 100 mL of normal saline acclimatized to room temperature. Do not shake or squeeze the IV bag.
- If partial vial is used, do not save or reuse the unused drug for later administration. Discard unused portions.
- Do not freeze or refrigerate reconstituted solution and administer within 4 hours or discard [see How Supplied/Storage and Handling (16)].
Erwinase is not a cytotoxic medicinal product (such as vincristine or methotrexate) and does not require the special precautions needed for manipulating such agents. It should be handled in the same way as other therapeutic enzymes such as hyaluronidase.
The solution should be administered within 15 minutes of reconstitution. If a delay of more than 15 minutes between reconstitution and administration is unavoidable, the solution should be withdrawn into a glass or polypropylene syringe for the period of the delay.
Any unused product or waste material should be disposed of in accordance with local requirements.
The solution should be used within 8 hours.
2.3 Administration Instructions
Administer ERWINAZE in a setting with resuscitation equipment and other agents necessary to treat anaphylaxis.
ERWINAZE solution can be administered by intramuscular injection or by intravenous infusion.
- For intramuscular use, limit the volume of reconstituted ERWINAZE at a single injection site to 2 mL; if reconstituted dose to be administered is greater than 2 mL, use multiple injection sites.
- For intravenous use, infuse ERWINAZE in 100 mL of normal saline over 1 to 2 hours. Do not infuse other intravenous drugs through the same intravenous line while infusing ERWINAZE.
Method of administration
Erwinase solution can be given by intravenous infusion or intramuscular injection.
For IM injection the volume of reconstituted solution administered at a single injection site should not exceed 2 mL. Multiple injection sites should be used if this volume is exceeded.
For IV infusion, the reconstituted solution should be further diluted in 100 mL of normal saline and administered over 1 to 2 hours.
For further instructions on reconstitution of the medicinal product before administration, see section 6.6.
Description
3 DOSAGE FORMS AND STRENGTHS
For injection: 10,000 International Units as a lyophilized powder in a single-dose vial for reconstitution
11 DESCRIPTION
ERWINAZE (asparaginase Erwinia chrysanthemi) contains an asparagine specific enzyme derived from Erwinia chrysanthemi. L-asparaginase is a tetrameric enzyme consisting of four identical subunits, each having a molecular weight of about 35 kDa. The activity of ERWINAZE is expressed in terms of International Units.
ERWINAZE is supplied as a sterile, lyophilized, white powder in vials. Each vial contains 10,000 International Units of asparaginase Erwinia chrysanthemi, and the following inactive ingredients: glucose monohydrate (5.0 mg), sodium chloride (0.5 mg).
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Crisantaspase (L-asparaginase from Erwinia chrysanthemi), 10,000 International units/vial.
For a full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Powder for solution for injection/infusion.
White lyophilised powder in a vial.
6.1 List of excipients
Sodium Chloride (0.5 mg)
Glucose Monohydrate (5.0 mg)
Contraindications
4 CONTRAINDICATIONS
ERWINAZE is contraindicated in patients with a history of:
- Serious hypersensitivity reactions to ERWINAZE, including anaphylaxis
- Serious pancreatitis with prior L-asparaginase therapy
- Serious thrombosis with prior L-asparaginase therapy
- Serious hemorrhagic events with prior L-asparaginase therapy
4.3 Contraindications
- History of severe hypersensitivity to the active substance or to any of the excipients listed in section 6.1
- Current or past severe pancreatitis associated with L‑asparaginase therapy
- Current pancreatitis not associated with L‑asparaginase therapy
Precautions
5 WARNINGS AND PRECAUTIONS
4.4 Special warnings and precautions for use
In order to improve traceability of biological medicinal products, the tradename and batch number of the administered product should be clearly recorded (or stated) in the patient file.
5.1 Hypersensitivity Reactions
Grade 3 and 4 hypersensitivity reactions after the use of ERWINAZE have occurred in 5% of patients in clinical trials [see Adverse Reactions (6.1)].
Hypersensitivity reactions
Administration of Erwinase can cause hypersensitivity reactions (infusion/injection reactions), including reactions presenting as anaphylaxis.
Severe reactions are common.
Reactions have occurred following the first or subsequent administrations.
There is little or no cross-reactivity between crisantaspase and E. coli-derived L-asparaginase.
Reactions include
- reactions limited to the area at or near the site of IM or IV administration, and
- other reactions, including
- reactions with symptoms consistent with an anaphylactic reaction, and
- reactions accompanied by fever (see section 4.8).
Reactions can begin during or immediately following administration. In the majority of patients, local and non-local reactions occur within the first 24 hours. Later onset of reactions has been reported two days or later after IM administration.
Administer this product in a setting with resuscitation equipment and other agents necessary to treat anaphylaxis. If a serious hypersensitivity reaction occurs, discontinue ERWINAZE and initiate appropriate therapy.
Facilities should be made available for management of an anaphylactic reaction, should it occur, during administration. If a severe reaction occurs, Erwinase must be discontinued (see section 4.3).
Careful observation is required on re-exposure to L-asparaginase after any time interval (e.g. between induction and consolidation), which may increase the risk of anaphylactic and hypersensitivity reactions occurring.
5.2 Pancreatitis
Pancreatitis has been reported in 4% of patients in clinical trials [see Adverse Reactions (6.1)].
Evaluate patients with symptoms compatible with pancreatitis to establish a diagnosis. Discontinue ERWINAZE for severe or hemorrhagic pancreatitis manifested by abdominal pain > 72 hours and amylase elevation ≥ 2.0 x ULN. Severe pancreatitis is a contraindication to additional asparaginase administration. In the case of mild pancreatitis, hold ERWINAZE until the signs and symptoms subside and amylase levels return to normal. After resolution, treatment with ERWINAZE may be resumed.
Pancreatitis
Treatment with L-asparaginase, including Erwinase, can cause pancreatitis. L-asparaginase-induced pancreatitis can be limited to biochemical and/or radiologic manifestations, progress to pancreatitis with clinical symptoms, and be severe (see section 4.8).
Fatal outcome of pancreatitis due to L-asparaginase products, including Erwinase, has been reported.
Patients must be closely monitored for signs and symptoms of pancreatic toxicity and instructed to promptly report potential symptoms of pancreatitis. If pancreatitis is suspected based on clinical symptoms, serum amylase and lipase should be determined. In patients treated with L-asparaginase, increases of serum amylase and lipase may be delayed, mild or absent.
Erwinase must be permanently discontinued in case of severe pancreatitis (see section 4.3). Hypertriglyceridemia, if marked, can contribute to the development of pancreatitis (see section 4.8).
There have been isolated reports of first onset of clinical pancreatitis and detection of pancreatic pseudocyst formation several months after the last administration of L-asparaginase. Patients must be monitored for late-occurring signs of pancreatitis.
Development of chronic pancreatitis as well as persistent pancreatic insufficiency (exocrine insufficiency with, e.g., malabsorption; persistent glucose intolerance/diabetes mellitus) has been reported with L- asparaginase treatment.
5.3 Glucose Intolerance
Glucose intolerance has been reported in 5% of patients receiving ERWINAZE in clinical trials [see Adverse Reactions (6.1)]. In some cases, glucose intolerance may be irreversible. Monitor glucose levels in patients at baseline and periodically during treatment. Administer insulin therapy as necessary in patients with hyperglycemia.
Glucose Intolerance
Treatment with L-asparaginase, including Erwinase, can cause glucose intolerance and potentially severe hyperglycemia.
In some patients, ketoacidosis has been reported.
Patients must be monitored for developing hyperglycemia and potential complications.
Administration of insulin and possibly discontinuation of L‑asparaginase treatment may be necessary to manage hyperglycemia.
5.4 Thrombosis and Hemorrhage
Serious thrombotic events, including sagittal sinus thrombosis and pulmonary embolism have been reported with both E. coli and Erwinia-derived L-asparaginase therapy. The following coagulation proteins were decreased in the majority of patients after a 2-week course of ERWINAZE by intramuscular administration: fibrinogen, protein C activity, protein S activity, and anti-thrombin III.
Discontinue ERWINAZE for a thrombotic or hemorrhagic event until symptoms resolve; after resolution, treatment with ERWINAZE may be resumed.
Coagulation Disorders
Administration of L-asparaginase, including Erwinase, leads to decreased synthesis of coagulant, anticoagulant, and fibrinolytic proteins, abnormal coagulation times, and clinical coagulation abnormalities that can cause serious thromboembolic and bleeding events (see section 4.8).
Routine clotting screening should be performed before treatment initiation and monitored during treatment. Preventive measures must be considered.
If significant symptomatic coagulopathy occurs in addition to other clinically indicated interventions withhold Erwinase treatment until resolved. Treatment may then continue according to protocol, if the benefit of continued administration is considered to outweigh the risk from re-exposure.
Adverse reactions
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the label:
- Hypersensitivity reactions [see Warnings and Precautions (5.1)]
- Pancreatitis [see Warnings and Precautions (5.2)]
- Glucose intolerance [see Warnings and Precautions (5.3)]
- Thrombosis and hemorrhage [see Warnings and Precautions (5.4)]
4.8 Undesirable effects
a. Summary of the safety profile
The two most frequent adverse reactions are:
• Hypersensitivity, including urticaria, fever, arthralgia angioedema, bronchospasm, hypotension or even anaphylactic shock. In case of severe systemic hypersensitivity reaction, treatment should be discontinued immediately and withdrawn.
• Coagulation abnormalities (e.g. thromboses), due to protein synthesis impairment, are the second most frequent class of adverse reactions. Thromboses of peripheral, pulmonary or central nervous system blood vessels have been reported, potentially fatal or with residual delayed affects dependent upon the location of the occlusion. Other risk factors contributing to coagulation abnormalities include the disease itself, concomitant steroid therapy and central venous catheters.
Undesirable effects are generally reversible.
6.1 Clinical Trials Experience
Because clinical trials are conducted under controlled, but widely varying conditions, adverse reaction rates observed in clinical trials of ERWINAZE cannot be directly compared to rates in the clinical trials of other drugs and may not reflect the rates observed in practice.
The data presented below are based on information collected from Study 1, a single-arm, multi-center, open-label, safety and clinical pharmacology trial (intramuscular administration), the ERWINAZE Master Treatment Protocol (EMTP), an expanded access program (both intramuscular, intravenous, and other or unknown administration), and Study 2, a single-arm, multi-center, open-label, pharmacokinetic (PK) study trial of intravenous administration of ERWINAZE.
Study 1 enrolled 58 patients treated on National Cancer Institute (NCI)-sponsored cooperative group ALL protocols who were unable to continue to receive pegaspargase due to hypersensitivity reactions.
Patients received 6 doses of ERWINAZE 25,000 International Units/m2 intramuscularly on a Monday, Wednesday, and Friday schedule as a replacement for each scheduled dose of pegaspargase remaining on their original treatment protocol. The Study 1 population included patients with a median age of 11 years (2 to 18 years); 59% were male, 78% were White, 10% were Black/African American, 5% were Asian, and 7% were other or unknown. A total of 35% were Hispanic or Latino. In Study 1, the number of ERWINAZE courses ranged from 1 to 9. In this study, 76% (44 of 58) completed all planned therapy.
Fourteen (24%) patients stopped therapy prior to completion; seven due to allergic reactions, five due to physician or patient choice, one due to disease progression, and one due to discontinuation during frontline protocol. All other chemotherapy was continued according to the patient’s prescribed treatment regimen [see Clinical Studies (14)].
Study 2 enrolled 30 patients [29 were being treated for ALL and one for lymphoblastic lymphoma (LBL)] following allergy to native E. coli asparaginase or pegaspargase. Patients received ERWINAZE 25,000 International Unit/m /dose, administered by intravenous infusion on a Monday, Wednesday, and Friday schedule (6 doses) as a replacement for doses remaining on their original treatment plan. The Study 2 population included patients with a median age of 7 years (1 to 17 years); 63% were male, 27% were Hispanic or Latino, 83% were White, 3% were Black/African American, 7% were Asian, and 7% were other (American Indian, Alaska Native or Indian) [see Clinical Studies (14)].
The EMTP trial enrolled 1368 patients with ALL or lymphoblastic lymphoma who received ERWINAZE after developing systemic hypersensitivity to an E. coli-derived asparaginase. Of these 1368 patients, safety data were received for 940 patients with a median age of 9 years (0 to 76 years), 63% were male, 91% with leukemia, 3% with lymphoma, and 6% with unknown disease information.
Patients received ERWINAZE according to several schedules, and treatment center specifications with doses that ranged from 20,000 to 25,000 International Units/m2. The route of administration was intramuscular n=852, intravenous n=29, other or unknown n=59. In the EMTP trial, the planned number of doses of ERWINAZE ranged from 3 to 48 doses. Seventy-eight percent of patients (693 of 893) were able to receive all planned doses to complete their prescribed treatment regimen.
In Study 1 and Study 2, safety information was prospectively and systematically collected. In Study 1, all Grades of adverse events were reported for the following adverse events of special interest: allergy, pancreatitis, coagulopathy (hemorrhage, thrombosis or infarct), hyperbilirubinemia, hyperglycemia, hyperlipidemia, ketoacidosis, and CNS events (hemorrhage, thrombosis or infarction, and cerebral venous thrombosis) and only Grade 3 and 4 events were reported for other adverse events. In Study 2 all adverse events of all Grades were prospectively collected. In the EMTP trial, safety data were derived from case report forms that collected adverse event information. The forms specifically requested information on occurrence of allergic reactions, thrombotic events, hemorrhagic events, hepatobiliary disorders, pancreatic disorders, and hyperglycemia.
The most common adverse reactions (incidence 1% or greater) with ERWINAZE treatment are systemic hypersensitivity, hyperglycemia, transaminases abnormal, fever, pancreatitis, local reactions, vomiting, nausea, thrombosis, hyperbilirubinemia, abdominal pain/discomfort, and diarrhea.
The incidence of non-hematologic, non-infectious, adverse events (all Grades) in Study 1, Study 2, and the EMTP trial is provided in Table 1.
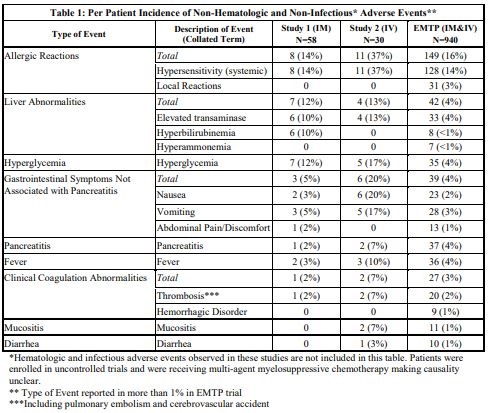
The incidence of Grade 3 or greater non-hematologic, non-infectious adverse reactions occurring with ERWINAZE in Study 1, Study 2 and EMTP trial is provided in Table 2.
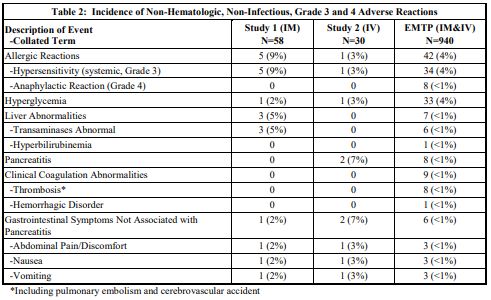
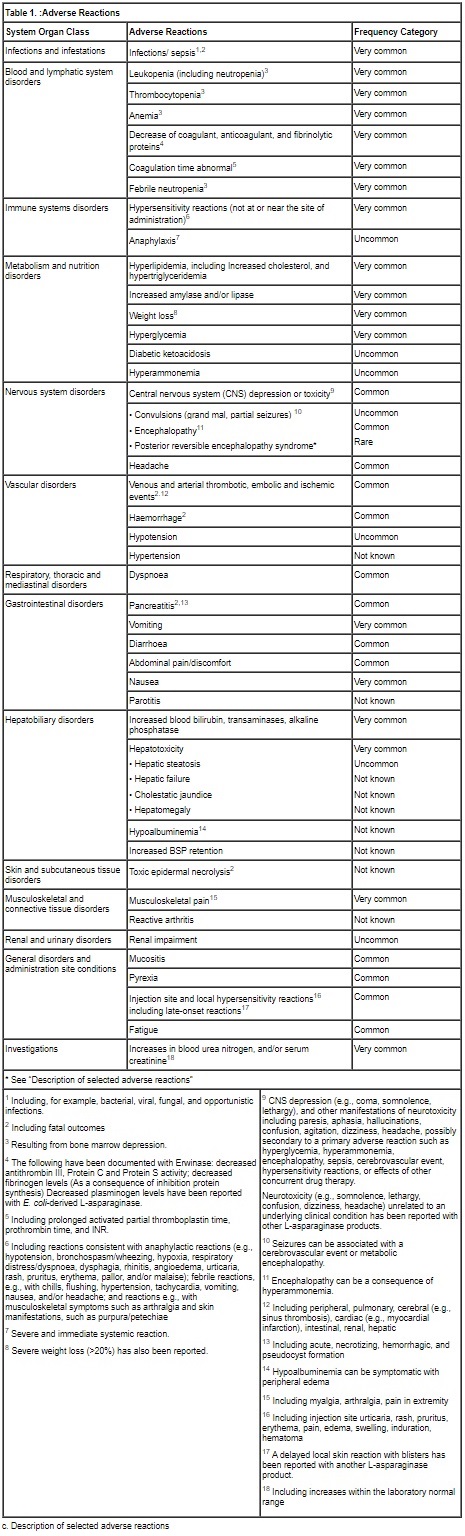
b. Tabulated list of adverse reactions
The adverse reaction data presented in Table 1 have been identified from 3 clinical studies (100EUSA12, ALL07P2, and Erwinase Master Treatment Protocol [EMTP]) with Erwinase in 1028 patients (primarily pediatric patients), the majority having acute lymphoblastic leukemia, as well as post-marketing experience with Erwinase and other L‑asparaginase preparations in pediatric and adult patients.
Some of the adverse reactions listed below are known to be associated with multi-agent chemotherapeutic regimens (e.g., reactions resulting from bone marrow depression, and infections), and the contributory role of Erwinase is not clear. In individual cases of other adverse reactions, other medicinal products of the regimen may have contributed.
Frequency definitions: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1000 to <1/100), rare (≥1/10000 to <1/1000) and very rare (<1/10000).When no valid estimate of the incidence rate for an adverse event from available data can be calculated, the frequency of such ADR has been classified as “Not known”.
Posterior reversible encephalopathy syndrome
In rare cases, a posterior reversible encephalopathy syndrome (PRES) has been observed during therapy with asparaginase-containing regimens.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in other studies or to other asparaginase Erwinia chrysanthemi products may be misleading.
In a study with ERWINAZE treatment by intramuscular administration (Study 1), 6 of 56 (11%) patients treated with ERWINAZE developed antibodies to ERWINAZE. Of these 6 anti-drug antibody (ADA) positive patients, one experienced a hypersensitivity reaction during Study 1 (2%, 1 of 56). None of these 6 patients had neutralizing antibodies.
In a study with ERWINAZE treatment by intravenous administration (Study 2), 4 of 30 (13.3%) patients treated with ERWINAZE developed anti-ERWINAZE antibodies. Of these 4 patients who developed anti-ERWINAZE antibodies, 3 experienced hypersensitivity reactions (10%, 3 of 30) during the study. None of these 4 patients had neutralizing antibodies.
The presence of ADA to ERWINAZE is associated with a higher risk of hypersensitivity reactions in patients who received ERWINAZE through intravenous infusion compared to intramuscular administration of ERWINAZE.
Immunogenicity
As with most therapeutic proteins, patients may potentially develop anti-drug antibodies (ADA) to crisantaspase.
Immunogenicity assays are highly dependent on the sensitivity and specificity of the assay and may be influenced by several factors such as: assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to crisantaspase with the incidence of antibodies to other products may be misleading.
In a study with Erwinase treatment by IM administration (Study ALL07P2), 6 of 56 (11%) patients treated with Erwinase developed antibodies to crisantaspase. Of these 6 ADA positive patients, one experienced a hypersensitivity reaction (2%, 1 of 56). None of these 6 patients had neutralising antibodies.
In a study with Erwinase treatment by IV administration (Study 100EUSA12), 4 of 30 (13.3%) patients treated with Erwinase developed anti-crisantaspase antibodies. Of these 4 patients, 3 experienced hypersensitivity reactions (10%, 3 of 30). None of these 4 patients had neutralising antibodies
Neutralising antibodies
As with other L-asparaginase preparations, development of specific neutralising antibodies has been reported with repeated dosing and is associated with reduced L-asparaginase activity.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.
Drug interactions
Note: The following information is provided in the USPI under section 12.3 Pharmacokinetics
Drug Interaction Studies
No formal drug interaction studies between ERWINAZE and other drugs have been performed
4.5 Interaction with other medicinal products and other forms of interaction
No formal medicinal product interaction studies have been performed.
Asparaginase must not be mixed with any other medicinal products prior to administration.
In addition concomitant use of L-asparaginase and medicinal products affecting liver function may increase the risk of a change in liver parameters (e.g. increase of ASAT, ALAT, bilirubin).
Since an indirect interaction between components of the oral contraception and asparaginase cannot be ruled out, oral contraceptives are not considered sufficiently safe in such clinical situation. Another method than oral contraception should be used in women of childbearing potential (see section 4.6).
• Methotrexate, cytarabine
L-asparaginase may diminish or abolish methotrexate’s and cytarabine’s effect on malignant cells; this effect persists as long as plasma asparagine levels are suppressed. Accordingly, do not use methotrexate or cytarabine with, or following L-asparaginase, while asparagine levels are below normal.
Alternatively, administration of L-asparaginase after methotrexate or cytarabine results in a synergistic effect. The extent to which these affect the overall effectiveness of established treatment protocols is not known.
• Prednisone
Concomitant use of prednisone and L-asparaginase may increase the risk of a change in clotting parameters (e.g. a decrease in fibrinogen and ATIII levels).
• Vincristine
Administration of vincristine concurrently with or immediately before treatment with L-asparaginase may be associated with increased toxicity and increased risk of anaphylaxis.
Special populations
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal reproduction studies, ERWINAZE can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, intramuscular administration of asparaginase Erwinia chrysanthemi to pregnant rats and rabbits during organogenesis at doses approximately 0.005-0.5 times the maximum recommended human dose resulted in structural abnormalities and embryo-fetal mortality (see Data). There are no available data on ERWINAZE use in pregnant women to evaluate the drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Advise pregnant women of the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2 to 4% and 15 to 20%, respectively.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of crisantaspase (Erwinia L-asparaginase) in pregnant women. Limited reports in humans of the use of E. coli asparaginase in combination with other antineoplastics during pregnancy did not provide sufficient data to conclude. However, based on effects on embryonal/foetal development shown in pre-clinical studies (see section 5.3),
Erwinase should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus.
Data
Animal Data
In embryofetal development studies, asparaginase Erwinia chrysanthemi was administered intramuscularly every other day during the period of organogenesis to pregnant rats (at 3000, 6000, or 12000 IU/m2) and rabbits (at 120, 300, or 480 IU/m2). In rats given 12000 IU/m2 (approximately 0.5 times the maximum recommended human dose), maternal toxicity of decreased body weight gain was observed, as well as a fetal finding of increased incidence of partially undescended thymic tissue.
In rabbits, maternal toxicity consisting of decreased body weight was observed at 480 IU/m2 (approximately 0.02 times the maximum recommended human dose). Increased post-implantation loss, a decrease in the number of live fetuses, and gross abnormalities (e.g., absent kidney, absent accessory lung lobe, additional subclavian artery, and delayed ossification) were observed at doses of ≥120 IU/m2 (approximately 0.005 times the maximum recommended human dose).
Fertility
There are no human data on the effect of crisantaspase on fertility. In rats, crisantaspase did not affect male and female fertility. However, a decrease in sperm count was observed in male rats (see section 5.3). The relevance of this finding to humans is not known.
8.2 Lactation
Risk Summary
There are no data on the presence of asparaginase Erwinia chrysanthemi in human or animal milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in the breastfed child, advise patients that breastfeeding is not recommended during treatment with ERWINAZE, and for 3 months after the last dose.
Breast feeding
It is not known whether crisantaspase (Erwinia L-asparaginase) is excreted in human breast milk. Potential serious adverse reactions may occur in nursing infants, therefore Erwinase should be discontinued during breast-feeding.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential before starting ERWINAZE treatment.
Contraception
Females
ERWINAZE can cause embryo-fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with ERWINAZE and for 3 months after the final dose. Since an indirect interaction between oral contraceptives and ERWINAZE cannot be ruled out, a method of contraception other than oral contraceptives should be used in women of childbearing potential.
Women of childbearing potential/Contraception in males and females
Women of childbearing potential should use effective contraception and avoid becoming pregnant while being treated with asparaginase-containing chemotherapy.
Since an indirect interaction between components of the oral contraception and asparaginase cannot be ruled out, oral contraceptives are not considered sufficiently safe in such clinical situation. A method other than oral contraceptives should be used in women of childbearing potential.
Men should use effective contraceptive measures and be advised to not father a child while receiving asparaginase.
The time period following treatment with asparaginase when it is safe to become pregnant or father a child is unknown. As a precautionary measure it is recommended to wait for three months after completion of treatment. However, treatment with other chemotherapeutic agents should also be taken into consideration.
8.4 Pediatric Use
The safety and effectiveness of ERWINAZE have been established in pediatric patients ages1 year and older as a component of a multi-agent chemotherapeutic regimen for the treatment of patients with acute lymphoblastic leukemia (ALL) who have developed hypersensitivity to E. coli-derived asparaginase and the information on this use is discussed throughout the labeling.
d. Pediatric population
Compared with children, the incidence of hepatic and pancreatic toxicities and of venous thromboembolic events may be increased in adolescents and young adults.
8.5 Geriatric Use
Clinical studies of ERWINAZE did not include geriatric patients.
e. Other special populations
No special individual populations of patients have been identified in which the safety profile differs from that defined above.
Overdosage
There are no corresponding clinical data for Erwinaze USPI
4.9 Overdose
There is no known antidote for asparaginase overdoses. No data are available on the elimination (peritoneal or by haemodialysis) of the product. Patients who accidentally receive an overdose of L-asparaginase should be monitored closely and receive any appropriate symptomatic and supportive treatment.
12 CLINICAL PHARMACOLOGY
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: other antineoplastic agents ATC code: L01XX02
12.1 Mechanism of Action
Asparaginase Erwinia chrysanthemi catalyzes the deamidation of asparagine to aspartic acid and ammonia, resulting in a reduction in circulating levels of asparagine. The mechanism of action of ERWINAZE is thought to be based on the inability of leukemic cells to synthesize asparagine due to lack of asparagine synthetase activity, resulting in cytotoxicity specific for leukemic cells that depend on an exogenous source of amino acid asparagine for their protein metabolism and survival.
Mechanism of action
L-asparaginase catalyses the deamination of asparagine to aspartic acid with the release of ammonia.
Asparagine is an amino acid found incorporated into most proteins, and protein synthesis is halted in its absence, thereby inhibiting RNA and DNA synthesis with a resulting halt to cellular proliferation.
As lymphoblastic cells are lacking asparagine synthetase activity they are dependent upon exogenous asparagine. The anti-tumour activity of L-asparaginase is a result of the sustained depletion of exogenous asparagine.
It has also been noted that asparaginase, in addition to its asparaginase activity, has significant glutaminase activity. It catalyses the deamination of glutamine in glutamic acid with the release of ammonia.
Glutamine may lead to alternative asparagine synthesis and therefore glutamine depletion may complement asparagine depletion. However, exact potential of this glutaminase activity remains unknown.
12.3 Pharmacokinetics
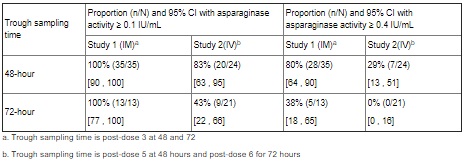
Based on a population PK model, the mean (%CV) half-life of intravenous ERWINAZE was 7.51 (23.9%) hours in contrast to a mean (%CV) half-life of 15.6 (20%) hours reported for intramuscular ERWINAZE. These differences in PK between intravenous and intramuscular ERWINAZE are reflected in the proportion of patients with 2-day and 3-day nadir serum asparaginase activity (NSAA) levels of asparaginase Erwinia chrysanthemi ≥ 0.1 or 0.4 IU/mL [see Clinical Studies (14)].
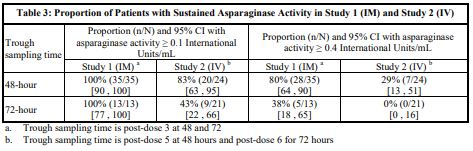
Following administration of ERWINAZE 25,000 International Units/m2 intramuscularly to 48 ALL patients aged ≥ 2 years to ≤ 18 years in Study 1 on a Monday, Wednesday, and Friday schedule for 6 doses, 100% of patients who completed Course 1 achieved NSAA levels ≥ 0.1 International Units/mL at either 48 hours (n=35) or 72 hours (n=13) post dose 3. Eighty percent (28/35) of those evaluated at 48 hours and 38% (5/13) evaluated at 72 hours had nadir serum asparaginase activity levels ≥ 0.4 International Units/mL [see Clinical Studies (14)].
Following intravenous administration of ERWINAZE 25,000 International Units/m2 to 24 evaluable patients (aged ≥ 1 year to ≤ 17 years) in Study 2 on a Monday, Wednesday, and Friday schedule, 83% (20/24) and 43% (9/21) of patients who completed Course 1 achieved NSAA levels ≥ 0.1 International Units/mL at 48 hours post-dose 5 and 72 hours post dose 6, respectively. Twenty-nine percent (7/24) of those evaluated at 48 hours and no patients (0/21) evaluated at 72 hours had nadir serum asparaginase activity levels ≥ 0.4 International Units/mL [see Clinical Studies (14)].
Drug Interaction Studies
No formal drug interaction studies between ERWINAZE and other drugs have been performed
5.2 Pharmacokinetic properties
Based on a population PK model, the mean (%CV) half-life of crisantaspase is 7.5 (24%) hours after intravenous infusion in contrast to 15.6 (20%) hours after intramuscular injection. L-asparaginase penetrates through to the cerebrospinal fluid to a small degree and is also found in lymph.
Serum trough asparaginase activity ≥ 0.1 U/mL has been demonstrated to correlate with asparagine depletion (asparagine < 0.4 mcg/mL or 3 μM) and to serum levels that predict clinical efficacy.
Clinical trials
Study 1 (AALL07P2) was a single-arm, multicentre, open-label, safety and clinical pharmacology trial, which enrolled ALL patients who were unable to continue to receive pegaspargase due to hypersensitivity reactions. The main outcome measure was the proportion of patients who achieved a serum trough asparaginase level ≥ 0.1 IU/mL, which correlates with asparagine depletion and predicts clinical efficacy. Patients received Erwinase 25,000 IU/m2 intramuscularly for two weeks (total 6 doses) as a replacement for each scheduled dose of pegaspargase.
Out of 58 patients enrolled, 48 were evaluable for the main outcome measure in the first treatment course. The median age was 11 years (2 to 18 years) and 59% were male.
Study 2 (100EUSA12) was a single-arm, multicentre pharmacokinetic study in patients with ALL/LBL who had developed hypersensitivity to native E. coli asparaginase, pegaspargase, or calaspargase pegol. Patients received Erwinase 25,000 IU/m2 intravenously 3 days per week for up to 30 weeks. The main outcome measure was the proportion of patients with 2-day nadir serum asparaginase activity (NSAA) levels after the fifth dose ≥ 0.1 IU/mL.
Out of 30 patients enrolled, 24 were evaluable for the main outcome measure in the first treatment course. The median age was 7 years (1-17 years) and 63% were male.
The results of the two studies are presented in the table below.
Proportion of patients with sustained asparaginase activity
Neutralising antibodies
As with other L-asparaginase preparations, development of specific neutralising antibodies has been reported with repeated dosing and is associated with reduced L-asparaginase activity.
Cerebrospinal fluid activity
After IM administration of 25,000 IU/m2 Erwinase per week for 16 weeks, CSF L-asparagine levels were undetectable 3 days after last administration in 5 of 8 children (62.5%), and in 2 of 8 children (25%) after both the 5th and 6th administration during reinforced re-induction therapy.
Carcinogenesis, Mutagenesis, Impairment of Fertility
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term carcinogenicity studies in animals have been performed with asparaginase Erwinia chrysanthemi. No studies that assess the mutagenic potential of asparaginase Erwinia chrysanthemi have been conducted.
In a fertility and early embryonic development study in rats, asparaginase Erwinia chrysanthemi had no effect on male or female fertility when administered intramuscularly at doses of up to 12000 IU/m2 (approximately 0.50 times the maximum recommended human dose) every other day for a total of 35 doses. Findings in males included decreased sperm count at doses of more than 3000 IU/m2 (approximately 0.12 times the maximum recommended human dose).
5.3 Preclinical safety data
Adverse reactions not observed in clinical studies, but seen in animals at exposure levels similar to clinical exposure levels and with possible relevance to clinical use were as follows:
Reproduction and development toxicity
Embryotoxicity studies with Erwinia L-asparaginase have given evidence of teratogenic potential in rabbits. In addition, pre-clinical experience with other asparaginase preparations has shown teratogenic potential in rats, mice and rabbits with doses in the therapeutic ranges.
In a fertility and early embryonic development study in rats, IM administration of crisantaspase had no effect on male and female fertility at doses approximately 50% of the recommended human dose (based on body surface area). However, a 12 to 15% decrease in sperm count was observed at doses approximately 12 to 50% of the recommended human dose.
Carcinogenicity
Non-clinical studies have not been conducted to evaluate the carcinogenic or mutagenic potential of crisantaspase. Crisantaspase is an enzyme for which the structure and well documented activity do not suggest any carcinogenic or mutagenic potential.
Clinical data
14 CLINICAL STUDIES
The efficacy of ERWINAZE for the treatment of patients with acute lymphoblastic leukemia (ALL) who have developed hypersensitivity to E. Coli-derived asparaginase as a component of a multi-agent chemotherapeutic regimen was established in Study 1, a single-arm, multi-center, open-label, safety and clinical pharmacology trial. Additional safety data were obtained in the ERWINAZE Master Treatment Protocol (EMTP), an expanded access program [see Adverse Reactions (6)]. Study 1 enrolled patients treated on National Cancer Institute (NCI)-sponsored cooperative group ALL protocols who were unable to continue to receive pegaspargase due to hypersensitivity reactions. The main outcome measure was determination of the proportion of patients who achieved a serum trough asparaginase level greater than or equal to 0.1 International Units/mL. Serum trough asparaginase activity ≥ 0.1 International Units/mL has been demonstrated to correlate with asparagine depletion (asparagine < 0.4 mcg/mL or 3 μM) and to serum levels that predict clinical efficacy. Patients received ERWINAZE 25,000 International Units/m2 intramuscularly for two weeks (total 6 doses) as a replacement for each scheduled dose of pegaspargase remaining on their original treatment protocol.
Fifty-eight patients were enrolled in Study 1, of these 48 were evaluable for the main outcome measure based on availability of pharmacokinetic samples in Course 1. The median age was 11 years (2 to 18 years); 59% were male, 78% were White, 10% were Black/African American, 5% were Asian, and 7% were other or unknown. A total of 35% were Hispanic or Latino.
Study 1 met its main outcome measure of demonstrating that greater than 50% of the patients achieved the pre-specified trough asparaginase activity level of ≥ 0.1 International Units/mL at 48 or 72 hours following the third dose. Results for the main outcome measure and for an exploratory analysis using a higher cut-off (trough serum asparaginase activity levels ≥ 0.4 International Units/mL are presented in Table 3 [see Clinical Pharmacology (12.3)].
The safety and efficacy of intravenous administration were determined in Study 2 by characterizing the PK of a 25,000 International Units/m2 ERWINAZE dose given 3 days per week on a Monday, Wednesday, and Friday schedule for up to 30 weeks. This open-label, single-arm, multicenter PK study enrolled 30 patients. The main outcome measure was determination of the proportion of patients with 2-day NSAA levels (48-hour levels taken after the fifth dose) ≥ 0.1 International Unit/mL in the first 2 weeks of ERWINAZE treatment.
Of the thirty patients enrolled, 24 were evaluable for the main outcome measure based on the pharmacokinetic samples in Course 1. The median age was 7 years (1-17 years), 63% were male, 27% were Hispanic or Latino, 83% were White, 3% were Black/African American, 7% were Asian, and 7% were other (American Indian, Alaska Native, or Indian).
In Study 2, serum asparaginase activity of asparaginase Erwinia chrysanthemi was determined in 24 evaluable patients (aged ≥ 1 year to ≤17 years) following intravenous administration of ERWINAZE 25,000 International Units/m2. Five minutes after the 60-minute infusion in Course 1, the mean asparaginase activity level was 12.65 ± 3.16 International Unit/mL post-dose 1 and 12.11 ± 3.11 International Unit/mL post dose 4. The main study objective was met with an asparaginase activity level of ≥ 0.1 International Units/mL 48 hours after the fifth dose observed in 83% of patients. The 72-hour post dose 6 asparaginase activity level of ≥ 0.1 International Unit/mL was the secondary endpoint, with 43% of patients achieving this endpoint. Results are presented in Table 3 [see Clinical Pharmacology (12.3)].
5.2 Pharmacokinetic properties
Based on a population PK model, the mean (%CV) half-life of crisantaspase is 7.5 (24%) hours after intravenous infusion in contrast to 15.6 (20%) hours after intramuscular injection. L-asparaginase penetrates through to the cerebrospinal fluid to a small degree and is also found in lymph.
Serum trough asparaginase activity ≥ 0.1 U/mL has been demonstrated to correlate with asparagine depletion (asparagine < 0.4 mcg/mL or 3 μM) and to serum levels that predict clinical efficacy.
Clinical trials
Study 1 (AALL07P2) was a single-arm, multicentre, open-label, safety and clinical pharmacology trial, which enrolled ALL patients who were unable to continue to receive pegaspargase due to hypersensitivity reactions. The main outcome measure was the proportion of patients who achieved a serum trough asparaginase level ≥ 0.1 IU/mL, which correlates with asparagine depletion and predicts clinical efficacy. Patients received Erwinase 25,000 IU/m2 intramuscularly for two weeks (total 6 doses) as a replacement for each scheduled dose of pegaspargase.
Out of 58 patients enrolled, 48 were evaluable for the main outcome measure in the first treatment course. The median age was 11 years (2 to 18 years) and 59% were male.
Study 2 (100EUSA12) was a single-arm, multicentre pharmacokinetic study in patients with ALL/LBL who had developed hypersensitivity to native E. coli asparaginase, pegaspargase, or calaspargase pegol. Patients received Erwinase 25,000 IU/m2 intravenously 3 days per week for up to 30 weeks. The main outcome measure was the proportion of patients with 2-day nadir serum asparaginase activity (NSAA) levels after the fifth dose ≥ 0.1 IU/mL.
Out of 30 patients enrolled, 24 were evaluable for the main outcome measure in the first treatment course. The median age was 7 years (1-17 years) and 63% were male.
The results of the two studies are presented in the table below.
Proportion of patients with sustained asparaginase activity

Cerebrospinal fluid activity
After IM administration of 25,000 IU/m2 Erwinase per week for 16 weeks, CSF L-asparagine levels were undetectable 3 days after last administration in 5 of 8 children (62.5%), and in 2 of 8 children (25%) after both the 5th and 6th administration during reinforced re-induction therapy.
Supply, storage and handling
16 HOW SUPPLIED/STORAGE AND HANDLING
ERWINAZE is a sterile, white lyophilized powder supplied in a clear 3 mL glass vial. Each carton of ERWINAZE (NDC 57902-249-05) contains 5 vials. Each single vial (NDC 57902-249-01) contains 10,000 International Units asparaginase Erwinia chrysanthemi.
Store unused or unopened vials and cartons at 36°F to 46°F (2°C to 8°C). Protect from light. Do not use ERWINAZE after the expiration date on the vial.
6.5 Nature and contents of container
Type 1 clear neutral glass vials of 3 ml nominal capacity, closed with 13 mm halobutyl freeze-drying stoppers and aluminium overseals, containing a white lyophilised solid.
Pack size: 5 vials.
6.4 Special precautions for storage
Store in a refrigerator (+2°C to +8°C).
For storage conditions of the reconstituted medicinal product, see section 6.3.
17 PATIENT COUNSELING INFORMATION
Hypersensitivity
Inform patients of the risk of allergic reactions, including anaphylaxis. Instruct patients on the symptoms of allergic reactions and to seek medical advice immediately if they experience such symptoms [see Warnings and Precautions (5.1)].
Pancreatitis
Instruct patients on the risk of pancreatitis and to seek medical advice immediately if they experience abdominal pain [see Warnings and Precautions (5.2)].
Glucose Intolerance
Instruct patients on the risk of hyperglycemia and glucose intolerance. Advise patients to seek medical advice if they experience excessive thirst or any increase in the volume or frequency of urination [see Warnings and Precautions (5.3)].
Thrombosis
Instruct patients on the risk of thrombosis and hemorrhage and to seek medical advice immediately if they experience headache, arm or leg swelling, shortness of breath, and chest pain [see Warnings and Precautions (5.4)].
Pregnancy and Lactation
Advise female patients of reproductive potential to use effective contraceptive methods while receiving ERWINAZE and for at least 3 months after the last dose. Advise patients to notify their healthcare provider immediately in the event of a pregnancy or if pregnancy is suspected during ERWINAZE treatment [see Use in Specific Populations (8.3)]. Advise lactating women not to breastfeed during treatment with ERWINAZE and for at least 3 months after the last dose [see Use in Specific Populations (8.2)].
There is no corresponding section for Erwinase SmPC
Company information
Manufactured by:
Jazz Pharmaceuticals, Inc.
3170 Porter Drive, Palo Alto, CA 94304
U.S. License No. 1901
Erwinaze® is a registered trademark of Porton Biopharma Limited used under license by Jazz Pharmaceuticals.
PRINCIPAL DISPLAY PANEL
5 Vials NDC 57902-249-05
asparaginase Erwinia chrysanthemi Erwinaze®
For injection, Intramuscular or Intravenous Use
10,000 International Units per Vial
Rx Only Single Dose Vial. Discard unused portion.
7. MARKETING AUTHORISATION HOLDER
Porton Biopharma Limited Manor Farm Road
Porton Down, Salisbury, SP4 0JG United Kingdom
8. MARKETING AUTHORISATION NUMBER(S)
PL 44403/0002
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
First authorisation: 19 July 1985
Latest renewal: 25 May 2006
10. DATE OF REVISION OF THE TEXT
06/2020
-
PATIENT PACKAGE INSERT
Erwinase U.K. Package Leaflet, Information for the Patient
Package leaflet: Information for the patient Erwinase
10,000 IU, Powder for solution for injection/infusion Crisantaspase (L-asparaginase from Erwinia chrysanthemi)
Read all of this leaflet carefully before you start receiving this medicine because it contains important information for you.
- -
- Keep this leaflet. You may need to read it again.
- -
- If you have any further questions, ask your doctor or your pharmacist.
- -
- If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What Erwinase is and what it is used for
- What you need to know before you are given Erwinase.
- How Erwinase is given
- Possible side effects
- How to store Erwinase
- Contents of pack and other information
1. What Erwinase is and what it is used for
How does Erwinase work
Erwinase is an anti-blood-cell-cancer treatment from the pharmacotherapeutic group: Antineoplastic and immunomodulating agents. It works by lowering the levels of asparagine in your body, a substance the cancer cells need to survive.
What this medicine is used for
Erwinase is used for the treatment of a cancer of the white blood cells called Acute Lymphoblastic Leukaemia, in patients aged 4 months and above, who have developed allergic reactions to E.coli derived asparaginase.
Erwinase may be used alone or with other treatments.
2. What you need to know before you are given Erwinase
You should not be given Erwinase if:
■ you have previously had a severe allergic reaction to the active substance (Crisantapase- L- asparaginase from Erwinia chrysanthemi) or are allergic to any of the other ingredients of this medicine (see section 6).
■ You have, or have previously, had serious problems with your pancreas (severe pancreatitis) from using a medicine containing L-asparaginase
■ You have serious problems with your pancreas (severe pancreatitis)
Warnings and precautions
Talk to your doctor or pharmacist or nurse before taking Erwinase.
The following complications may arise during treatment with Erwinase:
- Serious life threatening allergic reactions. The hospital will have the necessary precautions in place to deal with such situations.
- Inflammation of the pancreas. If you experience abdominal pain this may be a sign of pancreatitis and should be reported to your doctor immediately. Fatal outcomes associated with pancreatitis have occurred.
- Increases in your blood sugar levels (Hyperglycemia). This can be controlled by receiving insulin sometimes even to fatal amounts (Hyperglycemia). This can be controlled by receiving insulin.
- Bleeding and blood clot disorders. During treatment your body’s ability to prevent excessive bleeding may be affected. In the case you experience any significant bleeding your treatment will be stopped. Your doctor will determine if, and when, treatment can be restarted.
- Liver dysfunctions can be caused or worsened. Discontinuation of Erwinase will be considered in the event of a severe reaction. Treatment can be restarted under close monitoring, but only once at least near complete recovery is achieved.
- Neurological disorders have been reported with fatal outcomes. Posterior reversible encephalopathy syndrome (characterised by headache, confusion, seizures and visual loss) may require blood- pressure lowering medicines and in case of seizure, anti-epileptic treatment.
- Kidney impairment due to high levels of a substance called uric acid in your blood from the chemotherapy.
- Reduced immune system that may increase your chances of an infection.
Monitoring during treatment with Erwinase
You will be monitored closely during and after treatment with Erwinase for:
- Allergic reactions
- Pancreas, kidney and liver functions
- Normal blood content
For traceability purposes your health care professional will record the product name and batch number for each dose of Erwinase you receive.
Other medicines and Erwinase
Tell your doctor or your pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription, particularly any of the following:
- Types of medicines used to treat cancer called ‘methotrexate’ or ‘cytarabine’ as they can affect the way Erwinase works.
- Prednisone which is used in cancer treatment may increase the risk of a change in clotting.
- Vincristine which is used in cancer treatment, this can increase the toxic effects of both medicinal products and increase the risk of anaphylaxis.
- Oral contraceptives.
Your doctor or your nurse will not mix Erwinase with other medicines in the same infusion.
However you will probably be given other medicines before, during or after Erwinase treatment as part of your course of therapy.
Pregnancy
If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Breastfeeding
You must not breast-feed your baby during your treatment with Erwinase, there may be a risk to the feeding child.
Fertility & Family planning
Potential for a decrease in male fertility cannot be out ruled.
When appropriate both men and women should use necessary contraceptive measures before, and for at least three months after treatment with Erwinase. Women should use a form of contraception other than oral contraceptives.
Driving and using machines
Erwinase can cause dizziness and drowsiness. This can affect your coordination and therefore your ability to drive and operate machinery.
Erwinase contains sodium and glucose
Erwinase contains the following ingredients:
■ sodium (less than 23 mg per dose). You can consider this medicine as essentially sodium free if you are on a salt-free or low-salt diet.
■ glucose. If you are diabetic, please note that each bottle of Erwinase contains 5 mg glucose.
3. How Erwinase is given
Dosage
Erwinase will only be given to you by health care professionals who are experienced in giving chemotherapy.
Your doctor will decide what dose to administer, how often you will be given Erwinase and for how long. It varies according to your body weight, your specific condition being treated, and your response to therapy.
Method of administration
Erwinase can be given to you in one of the following ways:
a) Into a vein (intravenous use). This may be given over 1 to 2 hours.
b) Into a muscle (intramuscular use).
If you are given more Erwinase than you should
If you are concerned that you have been given too much Erwinase, contact your doctor or another healthcare professional immediately.
If you think you have missed a dose of Erwinase
If you are concerned that you have missed a dose, contact your doctor or another healthcare professional immediately.
If you have any further questions on this product, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, Erwinase can cause side effects, although not everybody gets them. Erwinase will be given under strict medical supervision and your doctor may give you other medicines to treat these side effects. Most of the side effects will stop once you stop taking Erwinase.
Serious side effects
Tell your doctor immediately if you experience:
- Severe allergic reactions including blue discolouration of the lips and extremities (possible symptoms of hypoxia), swelling of the face and/or, shortness of breath, increased heart rate; wheezing, difficulty swallowing, hay fever like symptoms, rash, chills, flushing, high or low blood pressure, vomiting
- Redness, pain, swelling, bruising, or hardening of the skin at the site of the injection
- Damage to the Central Nervous System symptoms may include coma, encephalopathy, hallucinations, muscle weakness, confusion, dizziness, drowsiness, agitation, difficulty speaking
- Arm, leg or calf pain with or without swelling (symptoms of blood clots in the arm or leg), abdominal pain (symptoms of a blood clot in the area of the stomach, intestines, and kidneys) chest pain spreading to the arms, neck, jaw, back or stomach, feeling sweaty and breathless (which may be symptoms of a heart attack/myocardial infarction)
- Pain near your stomach or in your back (this may be inflammation of your pancreas)
- High blood sugar levels (hyperglycemia)
- Increased frequency of bleeding events including bruising even if you have not been injured
- Changes in liver functions (identified by laboratory testing)
Other side effects
Talk to your doctor if you get any of the following:
Very common side effects (may affect more than 1 in 10 people):
- -
- Infections, including blood infections caused by bacteria (sepsis). This may be due to low levels of white cells in your blood. You may experience fever, a rapid heart rate, confusion or a rash.
- -
- Decreases in normal blood content. Some of which may be due to reduced bone marrow activity.
- -
- Increase in blood fats, bilirubin, creatinine, urea levels and certain liver enzymes- your doctor will monitor these.
- -
- Weight loss
- -
- Generalised pain/Muscle pains
- -
- Nausea
Common (may affect up to 1 in 10 people) side effects include:
- -
- Difficulty breathing or stopping breathing
- -
- Mucositis (inflammation of the digestive tract)
- -
- Diarrhoea
- -
- Abdominal pain/discomfort
- -
- tiredness or headache
- -
- High temperature
Uncommon (may affect up to 1 in 100 people) side effects include:
- -
- life threatening complications of uncontrolled diabetes
- -
- High blood levels of ammonia
- -
- Fits (convulsions)
- -
- Build up of fats in the liver
- -
- Kidney dysfunction
Rare (may affect up to 1 in 1,000 people) side effects include:
- -
- Posterior reversible encephalopathy syndrome (a condition characterised by headache, confusion, seizures and visual loss).
Not known (frequency cannot be estimated from the available data)
- -
- Inflammation of the salivary gland at the back of the throat
- -
- Liver failure, increased mass of liver, jaundice
- -
- Decreased albumin levels in the blood causing water retention
- -
- Blistering and peeling of the skin (Toxic epidermal necrolysis)
- -
- Joint pain
Additional side effects in children and adolescents
Liver, pancreas and blood clotting side effects may be higher in adults compared to children.
Reporting of side effects
If you get any side effects, talk to your doctor or,pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via MHRA Yellow Card in Google Play or Apple App Store.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Erwinase
Keep this medicine out of the sight and reach of children.
Erwinase will not be used after the expiry date printed on the label after “EXP”. The expiry date refers to the last day of the month.
The unopened Erwinase vials will be stored in a refrigerator (between +2°C to +8°C) by the hospital.
After reconstitution, the product should be used within 15 minutes. If the delay is more than 15 minutes, the solution should be withdrawn into a glass or polypropylene syringe and used within 8 hours. The reconstituted product should be stored below 25°C.
6. Contents of the pack and other information
What Erwinase contains
The active substance is crisantaspase (L-asparaginase from Erwinia chrysanthemi). Each vial contains 10,000 International units of cristanaspase (L-asparaginase from Erwinia chrysanthemi).
The other excipients are sodium chloride (See section 2) and glucose monohydrate (See section 2).
What Erwinase looks like and contents of the pack
Erwinase is provided as a powder for solution for injection/infusion
It comes as a white lyophilized powder in a clear glass bottle with a rubber stopper and an aluminium seal. Each pack contains 5 glass bottles of powder.
Marketing Authorisation Holder and Manufacturer
Porton Biopharma Limited, Manor Farm Road,
Porton Down, Salisbury, SP4 0JG United Kingdom
This leaflet was last revised in June 2020
Erwinase is a registered trademark of Porton Biopharma Limited.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ERWINASE
asparaginase injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:81561-413 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPARAGINASE ERWINIA CHRYSANTHEMI (UNII: D733ET3F9O) (ASPARAGINASE - UNII:G4FQ3CKY5R) ASPARAGINASE ERWINIA CHRYSANTHEMI 10000 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) 5 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81561-413-05 5 in 1 CARTON 06/01/2021 1 NDC:81561-413-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 06/01/2021 Labeler - Porton Biopharma Limited (220514820) Establishment Name Address ID/FEI Business Operations Porton Biopharma Limited 220514820 manufacture(81561-413)