Label: JAFRA SUN SUNSCREEN BROAD SPECTRUM SPF 50- avobenzone, homosalate, octisalate, octocrylene spray
- NDC Code(s): 68828-296-01
- Packager: Jafra Cosmetics International
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
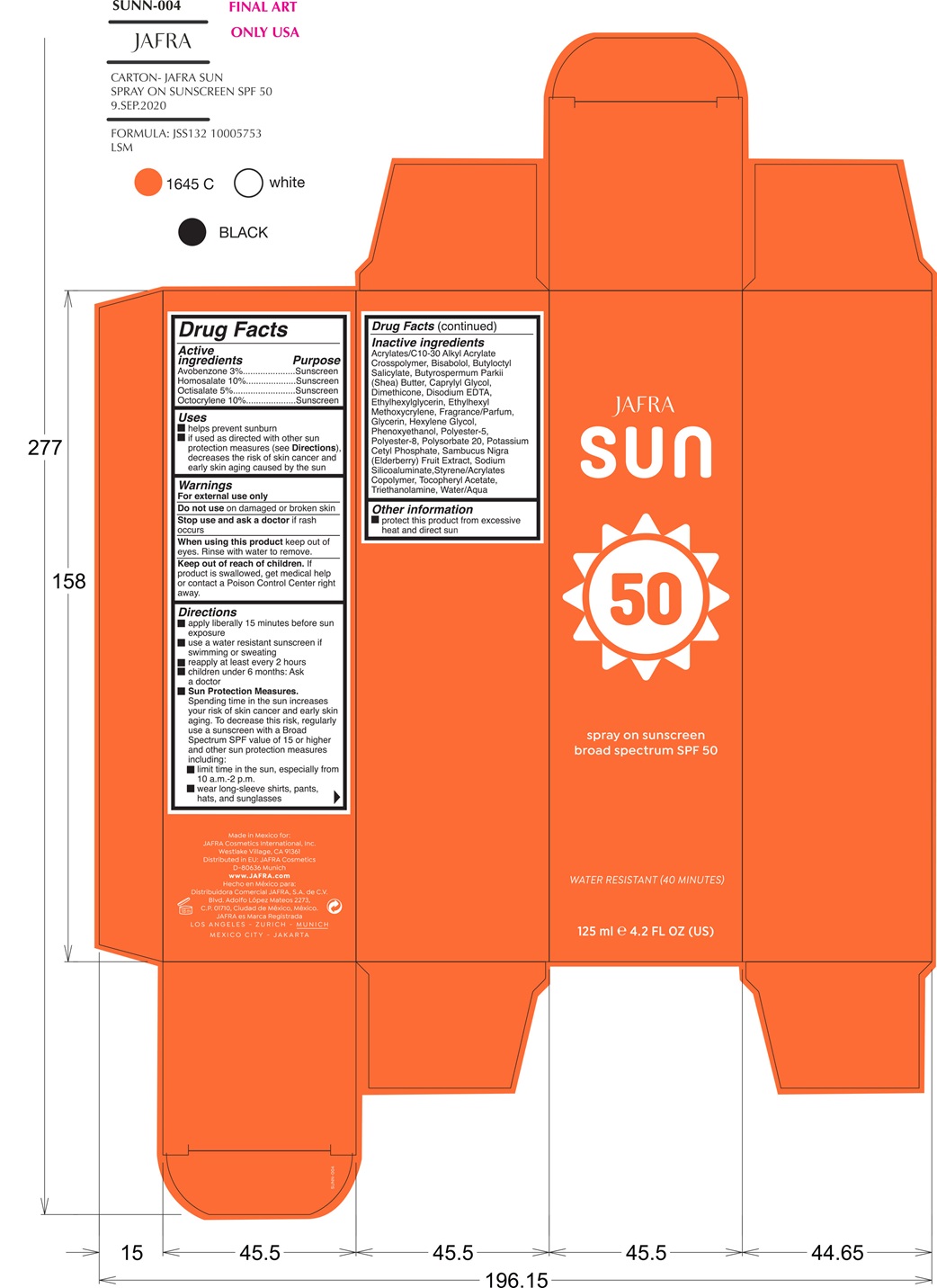
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water-resistant sunscreen if swimming or sweating
Reapply at least every 2 hours.
•Children under 6 months of age: ask a doctor.
- Sun Protection MeasuresSpending time in the sun increases your risk of
skin cancer and early skin aging. To decrease this risk, regularly use a
sunscreen with a Broad-Spectrum SPF value of 15 or higher and other sun
protection measures including:• limit your time in the sun, especially from 10
a.m. – 2 p.m.• wear long-sleeved shirts, pants, hats, and sunglasses
-
INACTIVE INGREDIENT
Inactive Ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Bisabolol, Butyloctyl Salicylate, Butyrospermum Parkii (Shea) Butter, Caprylyl Glycol, Dimethicone, Disodium EDTA, Ethylhexylglycerin, Ethylhexyl Methoxycrylene, Fragrance/Parfum, Glycerin, Hexylene Glycol, Phenoxyethanol, Polyester-5, Polyester-8, Polysorbate 20, Potassium Cetyl Phosphate, Sambucus Nigra (Elderberry) Fruit Extract, Sodium Silicoaluminate, Styrene/Acrylates Copolymer, Tocopheryl Acetate, Triethanolamine, Water/Aqua
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
JAFRA SUN SUNSCREEN BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate, octocrylene sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-296 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM ALUMINOSILICATE (UNII: 058TS43PSM) HEXYLENE GLYCOL (UNII: KEH0A3F75J) PHENOXYETHANOL (UNII: HIE492ZZ3T) TROLAMINE (UNII: 9O3K93S3TK) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) POLYSORBATE 20 (UNII: 7T1F30V5YH) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) DIMETHICONE (UNII: 92RU3N3Y1O) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) LEVOMENOL (UNII: 24WE03BX2T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) EUROPEAN ELDERBERRY (UNII: BQY1UBX046) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SHEA BUTTER (UNII: K49155WL9Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-296-01 125 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 01/14/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/14/2021 Labeler - Jafra Cosmetics International (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-296)