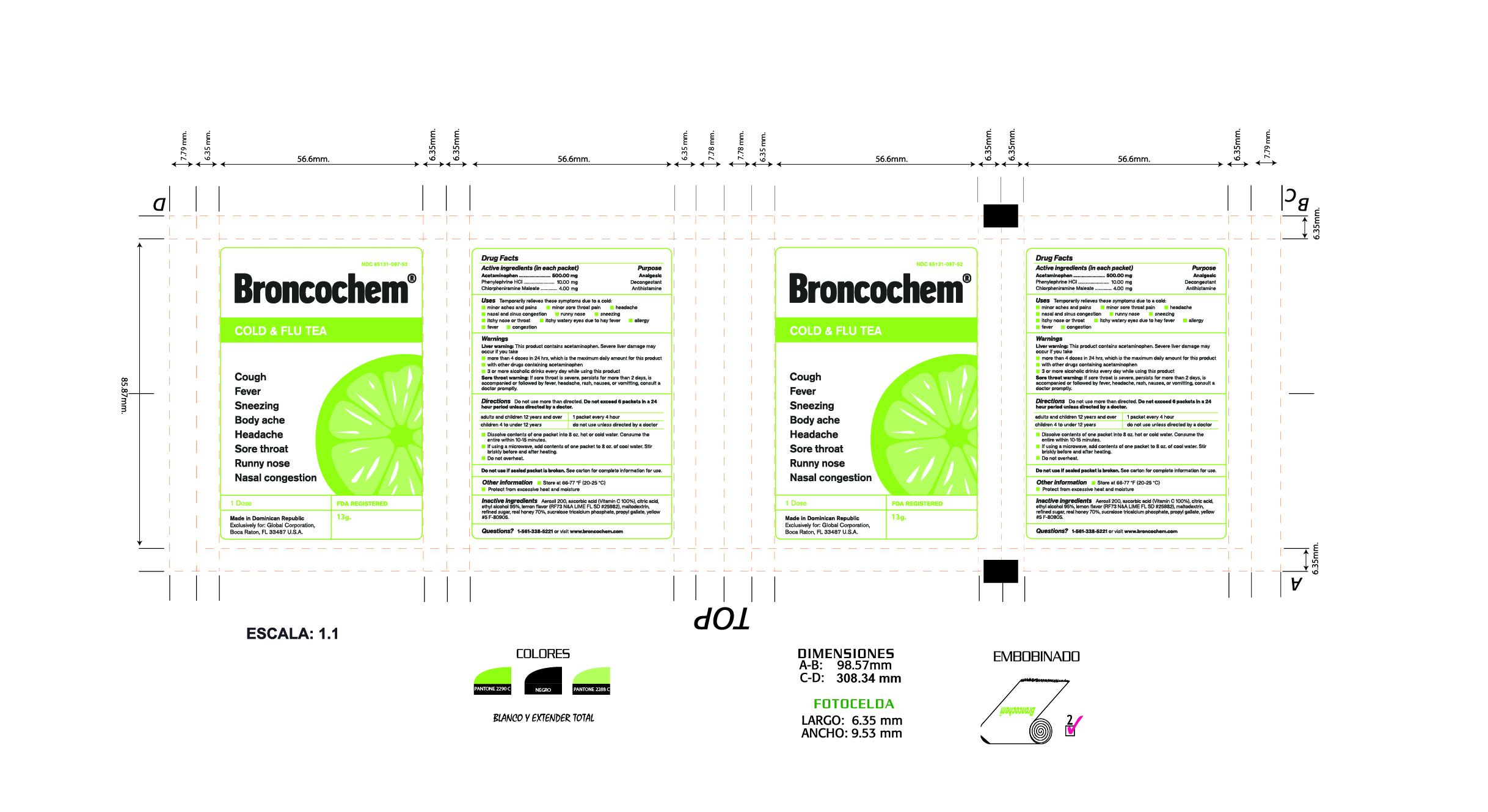
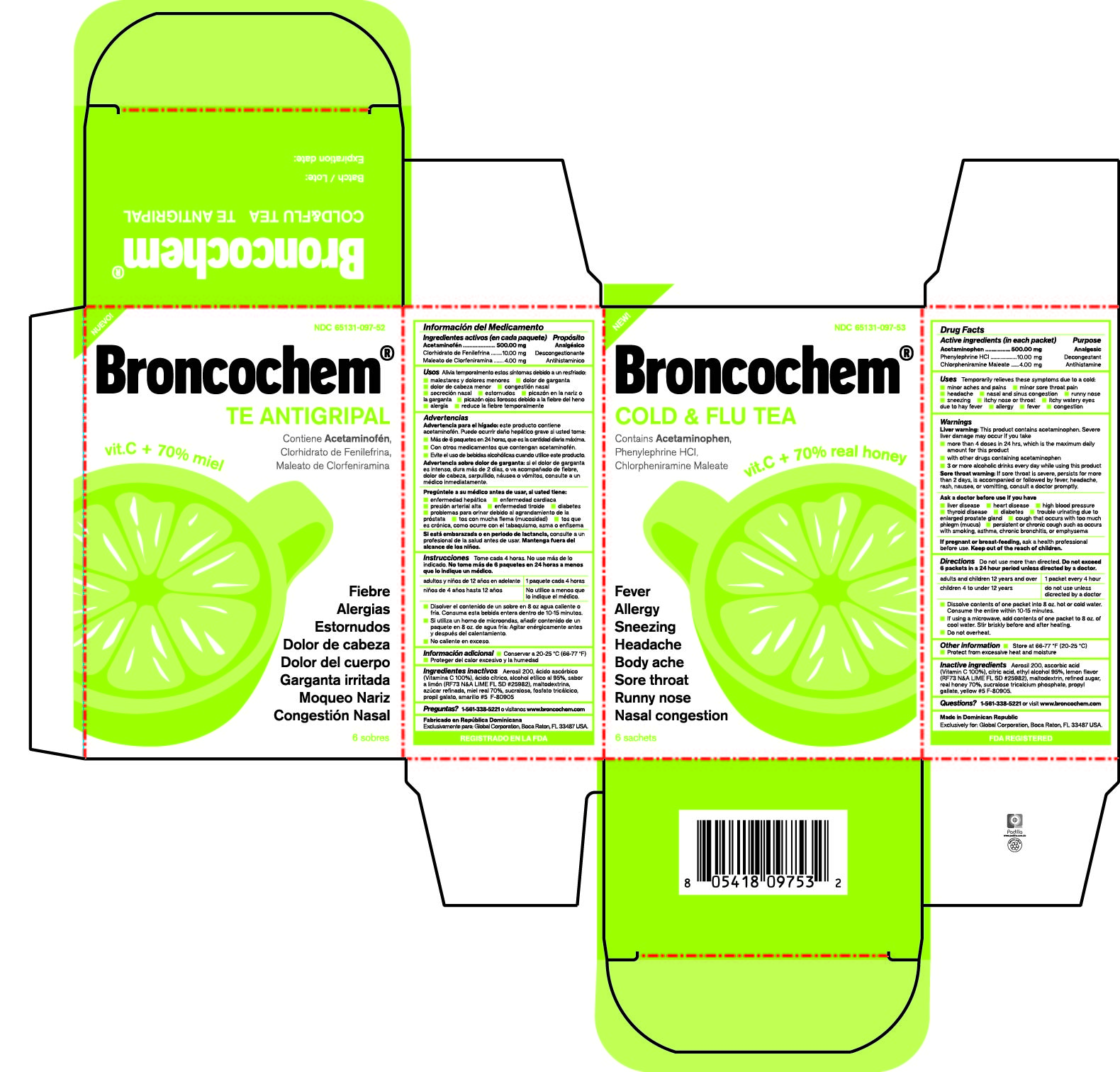
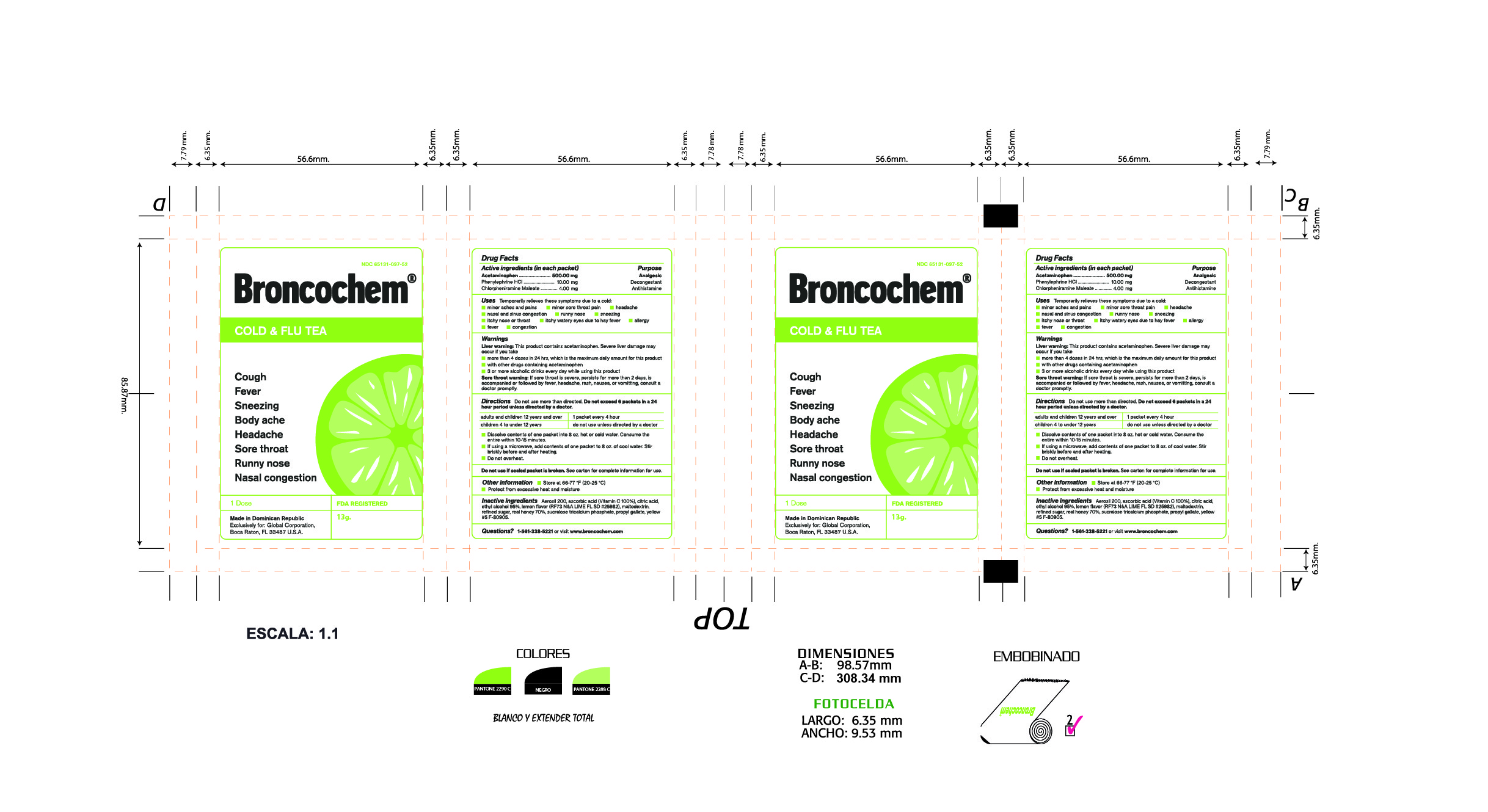
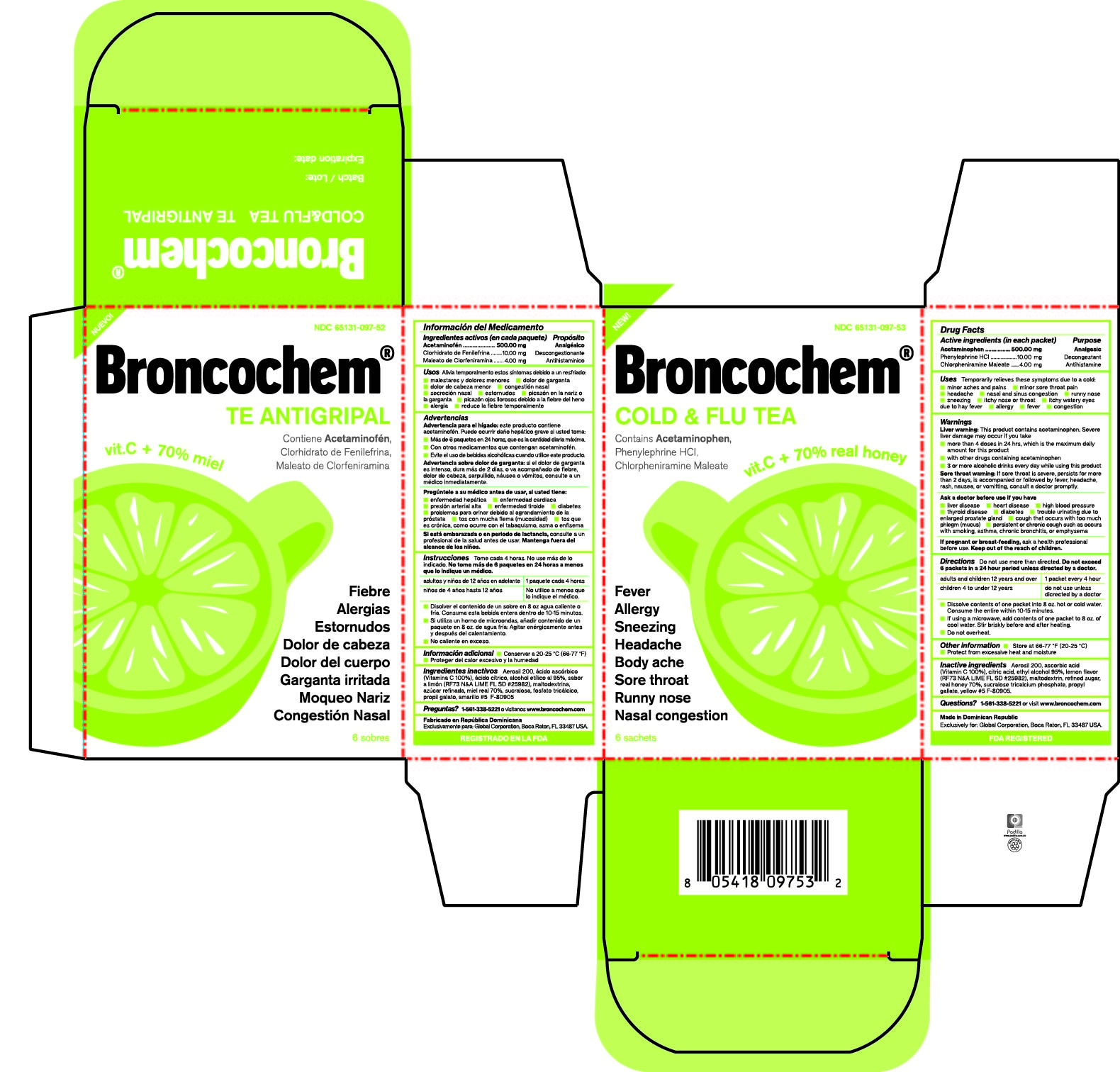
Label: BRONCOCHEM COLD AND FLU TEA- acetaminophen-phenylephrine hydrochloride-chlorpheniramine maleate granule, for solution
- NDC Code(s): 65131-097-12, 65131-097-52, 65131-097-53
- Packager: LABORATORIO MAGNACHEM INTERNATIONAL SRL
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 12, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Warnings
Liver warning:
This product contains acetaminophen, severe liver damage may occur if you take:
- More than 6 packets in 24 hours, which is the maximum daily amount.
- with other drugs containing acetaminophen.
- three (3) or more alcoholic drinks every day while using this product.
Sore Tthroat warning:
If sore throat is severe, persists for more than 2 days, is accompainied or followed by fever, headache, rash, nausea or vomiting consult a doctor promptly.
Active Ingredients
Acetaminophen.
Phenylephrine Hydrochloride.
Chlorpheniramine Maleate
Purpose
Pain reliever/fever reducer
Nasal decongestant
Antihistamine
Keep Out of the Reach of Children
In case of overdose get medical help or contact a Poison Control Center right away, prompt medical attention is critical for adults as well as for children even if you do not notice any signs symptoms.
Indications and Usage
Temporarily relieves these symptoms due to a cold:
- Minor aches and pains.
- Minor sore throat pain.
- Headache.
- Nasal and sinus congestion.
- Runny nose.
- Sneezing
- Itchy nose or throat.
- Itchy, watery eyes due to hay fever.
- Cough due to minor throat and bronchial irritation.
- Temporarily reduces fever.
Dosage and Administration
Do not use more than directed.
Take every 4 hours; do not take more than 6 packets in 24 hours unless directed by a doctor.
Children under 4 years of age-Do not ue.
Children from 4 to 12 years of age-Do not use unless directed by a doctor.
Adults and children 12 years of age and over-One packet
Dissolve contents of one packet into 8 oz of hot or cold water, consume entire drink within 10-15 minutes.
If using a microwave, add contents of one packet to 8 oz of cool wter stir briskly before and after heating.
Do not over heat.
- BAG IMAGE
- BOX IMAGE
- BOX IMAGE
-
INGREDIENTS AND APPEARANCE
BRONCOCHEM COLD AND FLU TEA
acetaminophen-phenylephrine hydrochloride-chlorpheniramine maleate granule, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65131-097 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg in 13 g CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg in 13 g Inactive Ingredients Ingredient Name Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) 10 mg in 13 g ASCORBIC ACID (UNII: PQ6CK8PD0R) 66 mg in 13 g SUCROSE (UNII: C151H8M554) 9483 mg in 13 g CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 1500 mg in 13 g HONEY (UNII: Y9H1V576FH) 100 mg in 13 g MALTODEXTRIN (UNII: 7CVR7L4A2D) 1000 mg in 13 g TRICALCIUM PHOSPHATE (UNII: K4C08XP666) 166.6 mg in 13 g SUCRALOSE (UNII: 96K6UQ3ZD4) 28.43 mg in 13 g SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 40 mg in 13 g PROPYL GALLATE (UNII: 8D4SNN7V92) 0.433 mg in 13 g ALCOHOL (UNII: 3K9958V90M) 0.001 mL in 13 g Product Characteristics Color yellow (FD&C YELLOW NO.5) Score Shape Size Flavor LIME (LIME (CITRUS)) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65131-097-53 6 in 1 BOX 09/28/2017 1 NDC:65131-097-52 13 g in 1 PACKAGE; Type 0: Not a Combination Product 2 NDC:65131-097-12 25 in 1 BOX 09/28/2017 2 NDC:65131-097-52 13 g in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 09/01/2013 Labeler - LABORATORIO MAGNACHEM INTERNATIONAL SRL (871446100) Registrant - LABORATORIO MAGNACHEM INTERNATIONAL SRL (871446100) Establishment Name Address ID/FEI Business Operations LABORATORIO MAGNACHEM INTERNATIONAL SRL 871446100 manufacture(65131-097)