Label: RECHARGE REGIMEN FOR BALANCING, BOOSTING AND DEFENDING HEALTHY-LOOKING SKIN- titanium dioxide, zinc oxide cream
- NDC Code(s): 14222-8000-1
- Packager: Rodan & Fields
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- After moisturizing, apply generously at least 15 minutes before sun exposure using fingertips. Smooth and blend evenly over entire face in an outward direction.
- Wear alone or under makeup to prime skin.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Children under 6 months of age: ask a doctor.
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- Wear long-sleeve shirts, pants, hats and sunglasses.
- Other Information
-
Inactive Ingredients
Isododecane, Cyclopentasiloxane, Dimethicone Crosspolymer, Calcium Sodium Borosilicate, Butyloctyl Salicylate, Jojoba Esters, Dimethicone, Glycerin, Sea Water/Maris Aqua/Eau De Mer, Hexyl Laurate, Acrylates/Dimethicone Copolymer, Water, Vaccinium Angustifolium (Blueberry) Fruit Extract, Carnosine, Sclareolide, Zinc PCA, Fragrance/Parfum, Silica, Dimethicone/Polyglycerin-3 Crosspolymer, Polyglyceryl-4 Isostearate, PEG-10 Dimethicone, Cetyl PEG/PPG-10/1 Dimethicone, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, Triethoxysilylethyl Polydimethylsiloxyethyl Hexyl Dimethicone, Stearic Acid, Biosaccharide Gum-4, Methyl Carboxymethylphenyl Aminocarboxypropylphosphonate, Isopropyl Titanium Triisostearate, Tocopherol, Sodium Citrate, Butylene Glycol, Propanediol, Dipropylene Glycol, Phenoxyethanol, Hexyl Cinnamal, Limonene, Linalool, Alumina, Iron Oxides (Cl 77499).
- Questions
-
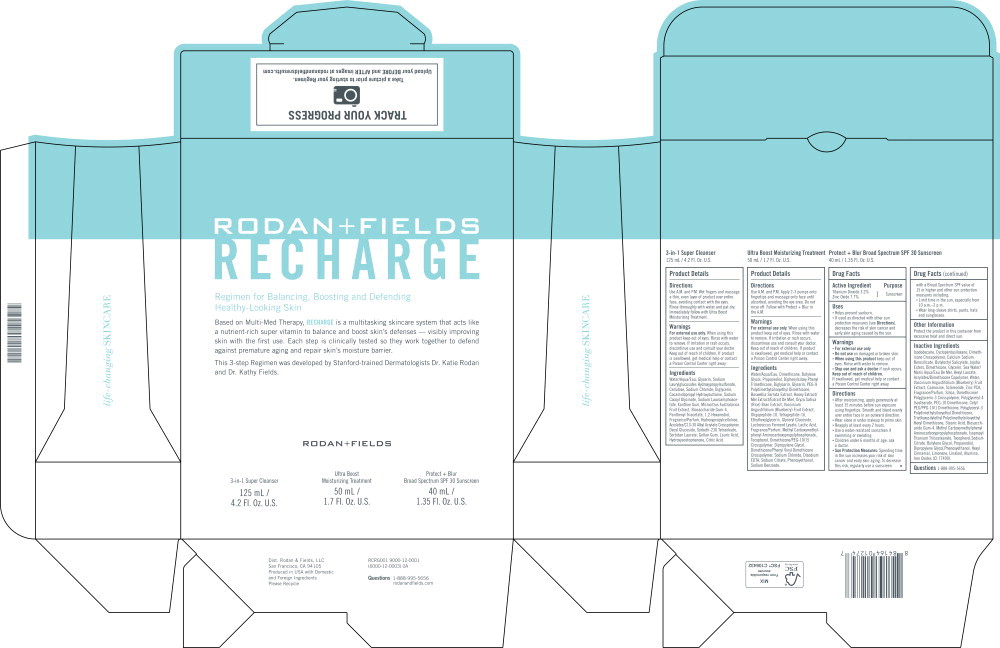
Principal Display Panel - Carton Label
RODAN + FIELDS
RECHARGERegimen for Balancing, Boosting and Defending
Healthy-Looking Skin
Based on Multi-Med Therapy, RECHARGE is a multitasking skincare system that acts like a nutrient-rich super vitamin to balance and boost skin's defenses — visibly improving skin with the first use. Each step is clinically tested so they work together to defend against premature aging and repair skin's moisture barrier.
This 3-step Regimen was developed by Stanford-trained Dermatologists Dr. Katie Rodan and Dr. Kathy Fields.
RDDAN + FIELDS
3-in-1 Super Cleanser
125 mL / 4.2 Fl. Oz. U.S.
Ultra Boost
Moisturizing Treatment
50 mL / 1.7 FI. Oz. U.S.
Protect + Blur
Broad Spectrum SPF 30 Sunscreen
40 mL / 1.35 FI. Oz. U.S.
-
INGREDIENTS AND APPEARANCE
RECHARGE REGIMEN FOR BALANCING, BOOSTING AND DEFENDING HEALTHY-LOOKING SKIN
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-8000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.032 g in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.077 g in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BOROSILICATE GLASS (UNII: BOJ6T9AR90) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) HYDROGENATED JOJOBA OIL/JOJOBA OIL, RANDOMIZED (IODINE VALUE 64-70) (UNII: 96YYQ5TK1K) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) HEXYL LAURATE (UNII: 4CG9F9W01Q) PROPANEDIOL (UNII: 5965N8W85T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) ZINC PIDOLATE (UNII: C32PQ86DH4) SCLAREOLIDE (UNII: 37W4O0O6E6) CARNOSINE (UNII: 8HO6PVN24W) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) PHENOXYETHANOL (UNII: HIE492ZZ3T) LINALOOL, (+/-)- (UNII: D81QY6I88E) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPROPYLENE GLYCOL (UNII: E107L85C40) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) LIMONENE, (+)- (UNII: GFD7C86Q1W) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 3) (UNII: G300307ZXP) TOCOPHEROL (UNII: R0ZB2556P8) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-8000-1 1 in 1 CARTON 09/20/2019 1 40 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/20/2019 Labeler - Rodan & Fields (051659584)