Label: HAND SANITIZING WIPE- hand sanitizing wipes cloth
-
Contains inactivated NDC Code(s)
NDC Code(s): 81339-200-30 - Packager: NeatGoods LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 10, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Keep out of reach of children
- Directions
- Inactive Ingredients
- Questions or Comments
-
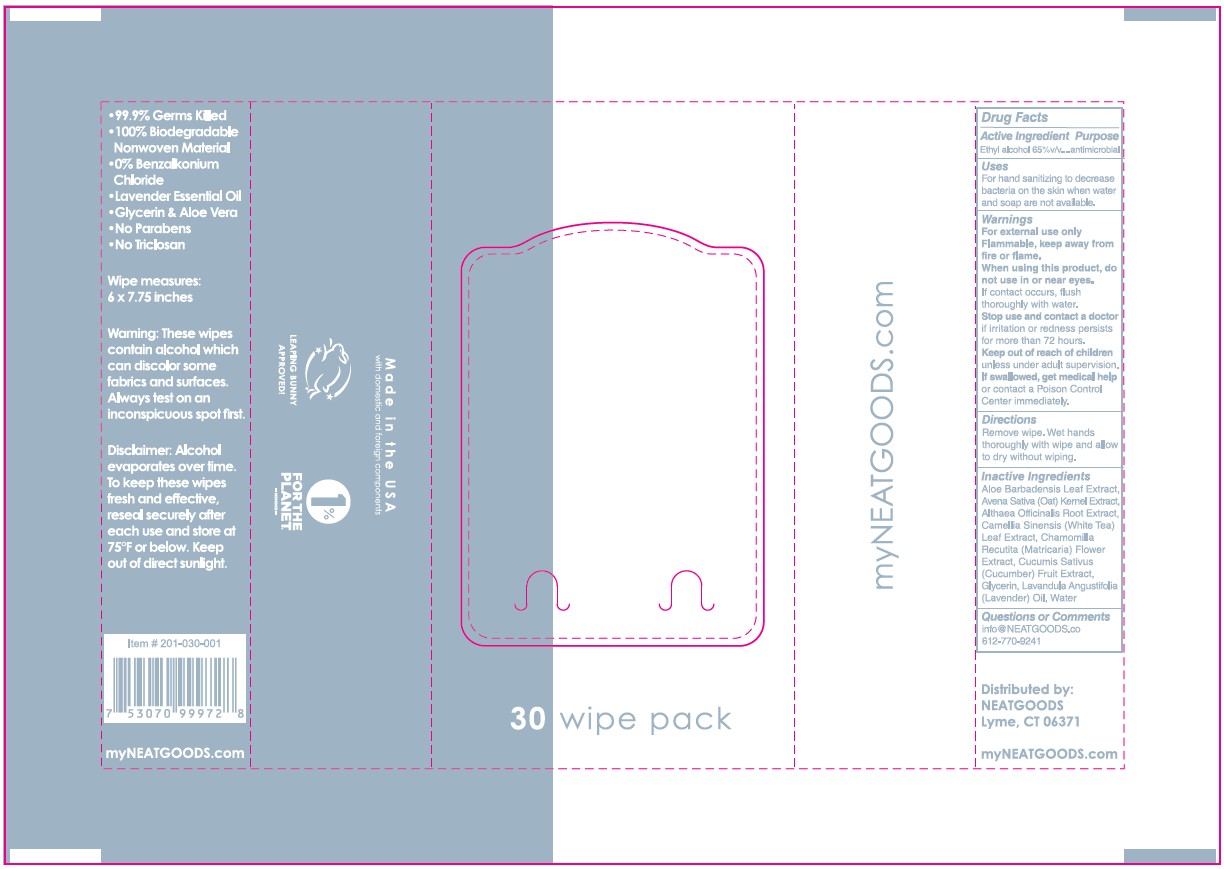
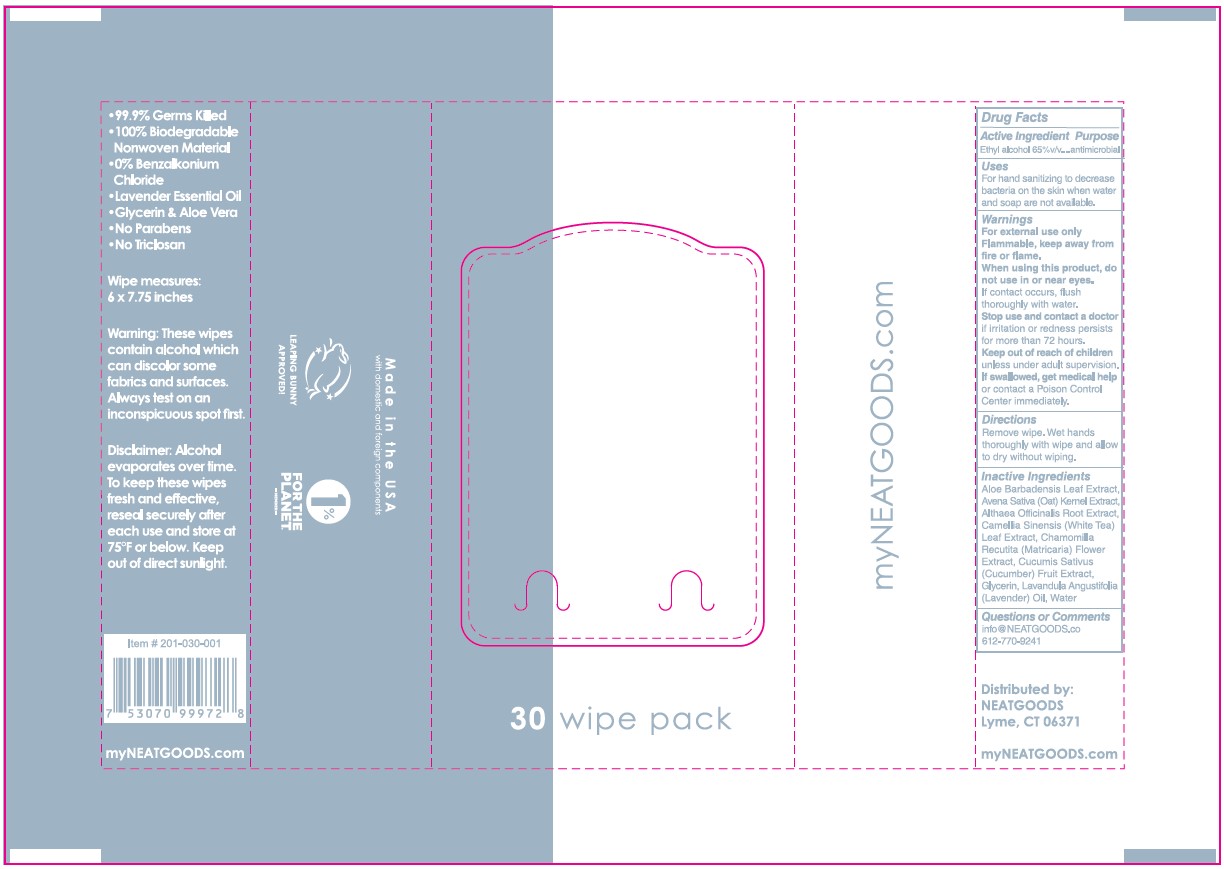
Label
NEATwipes TM
• 99.9% Germs Killed
• 100% Biodegradable Nonwoven Material
• 0% Benzalkonium Chloride
30 wipe pack
• Lavender Essential Oil
• Glycerin & Aloe Vera
• No Parabens
• No Triclosan
Wipe measures: 6 x 7.75 inches
Warning: These wipes contain alcohol which can discolor same fabrics
and surfaces. Always test an inconspicuous spot first.
Disclaimer: Alcohol evaporates over time. To keep these wipes fresh
and effective, reseal securely sfter each use and store at 75ºF or below.
Keep out of direct sunlight.
Item #: 201-030-001
7 53070 99972 8
my NEATGOODS.COM
Made in USA
with domestic and foreign components
LEAPING BUNNY APPROVED!
1% FOR THE PLANET. -MEMBER-
Distributed by:
NEATGOODS Lyme, CT 06371
from domestic and imported ingredients

-
INGREDIENTS AND APPEARANCE
HAND SANITIZING WIPE
hand sanitizing wipes clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81339-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65 mL in 100 mL Inactive Ingredients Ingredient Name Strength ALTHAEA OFFICINALIS ROOT (UNII: TRW2FUF47H) WATER (UNII: 059QF0KO0R) OAT (UNII: Z6J799EAJK) LAVENDER OIL (UNII: ZBP1YXW0H8) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CUCUMBER (UNII: YY7C30VXJT) GLYCERIN (UNII: PDC6A3C0OX) CHAMOMILE (UNII: FGL3685T2X) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81339-200-30 1 mL in 1 POUCH; Type 0: Not a Combination Product 12/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 12/28/2020 Labeler - NeatGoods LLC (080268970) Registrant - Diamond Wipes International, Inc. (161104729) Establishment Name Address ID/FEI Business Operations Diamond Wipes International, Inc. 161104729 manufacture(81339-200)