Label: SCHOLLS WELLNESS COMPANY LLC INGROWN TOENAIL- sodium sulfide gel
- NDC Code(s): 73469-7214-1
- Packager: Scholls Wellness Company LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- Purpose
- Use
- WARNINGS
- DO NOT USE
- ASK DOCTOR/PHARMACIST
- When using this product
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
adults and children 12 years and over:
wash affected area and dry thoroughly
place retainer ring on toe with slot over the area where the ingrown nail and the skin meet. Smooth ring down firmly.
cut open tip of tube on score mark. Apply enough gel product to fill the slot in the ring. Immediately replace cap on tube.
place round center section of bandage directly over the gel-filled ring to seal the gel in place. Smooth ends of bandage strip around toe.
repeat twice daily (morning and night) for up to 7 days until pain and discomfort is relieved or until the nail can be lifted out of the nail groove and easily trimmed.
children under 12 years: ask a doctor
- Other information
- INACTIVE INGREDIENT
- Questions
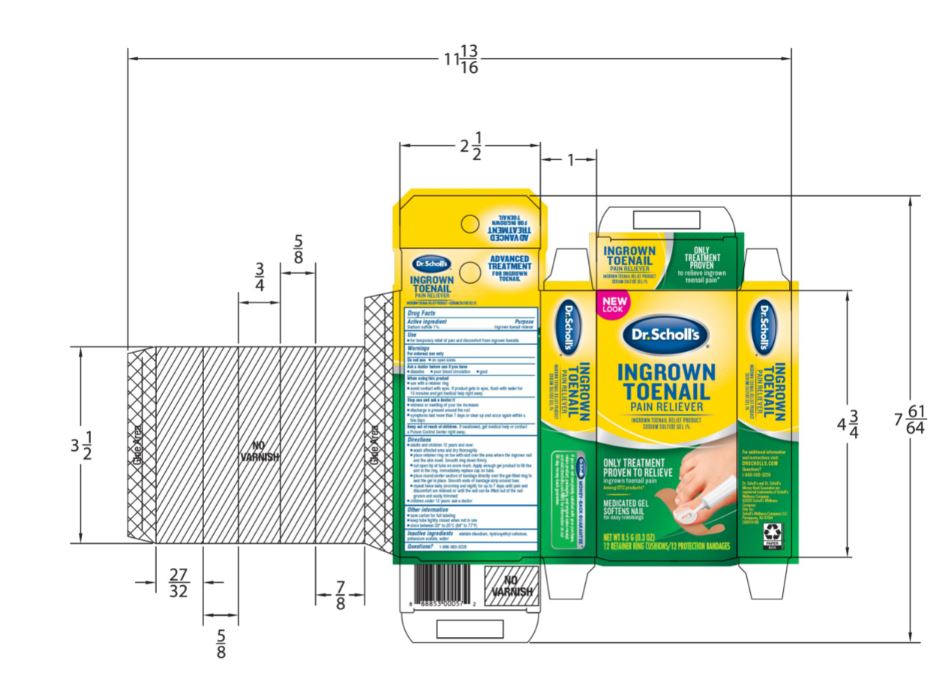
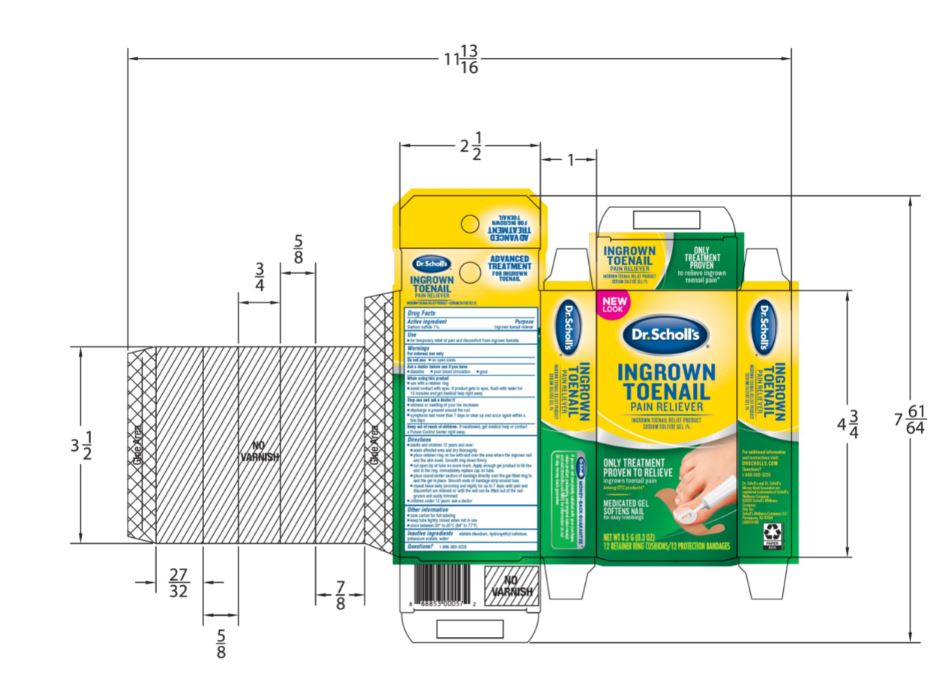
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SCHOLLS WELLNESS COMPANY LLC INGROWN TOENAIL
sodium sulfide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73469-7214 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM SULFIDE (UNII: YGR27ZW0Y7) (SULFIDE ION - UNII:G15I91XETI) SODIUM SULFIDE 1 g in 10 g Inactive Ingredients Ingredient Name Strength POTASSIUM ACETATE (UNII: M911911U02) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) Product Characteristics Color green Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73469-7214-1 1 in 1 CARTON 01/01/2021 08/31/2023 1 1 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358D 01/01/2021 Labeler - Scholls Wellness Company LLC (117174744) Registrant - Scapa Tapes North America, LLC (079995435)