Label: ITK BODY BLEMISH TREATMENT MIST- salicylic acid spray
- NDC Code(s): 71899-070-01
- Packager: MAESA, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
-
Warnings
- For external use only.
- Flammable: Do not use near heat, flame, or while smoking.
- When using this product and other topical acne medications at the same time or immediately following use of this product, increased dryness or irritation of the skin may occur. If this occurs, only one medication should be used unless directed by a doctor.
- Directions
- Inactive Ingredients
- Questions, Comments
- SPL UNCLASSIFIED SECTION
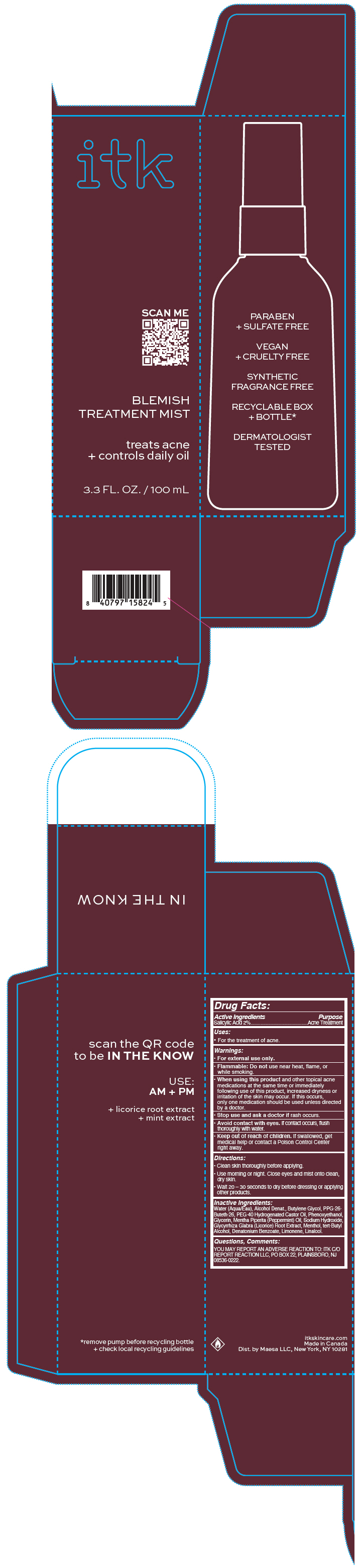
- PRINCIPAL DISPLAY PANEL - 100 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
ITK BODY BLEMISH TREATMENT MIST
salicylic acid sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71899-070 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 2 mg in 100 mL Inactive Ingredients Ingredient Name Strength DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PPG-26-BUTETH-26 (UNII: 2II1K6TZ4P) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERIN (UNII: PDC6A3C0OX) PEPPERMINT OIL (UNII: AV092KU4JH) SODIUM HYDROXIDE (UNII: 55X04QC32I) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71899-070-01 1 in 1 CARTON 08/01/2022 1 100 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M006 08/01/2022 Labeler - MAESA, LLC. (144282311) Establishment Name Address ID/FEI Business Operations Cosmetica Labs 255080491 MANUFACTURE(71899-070)