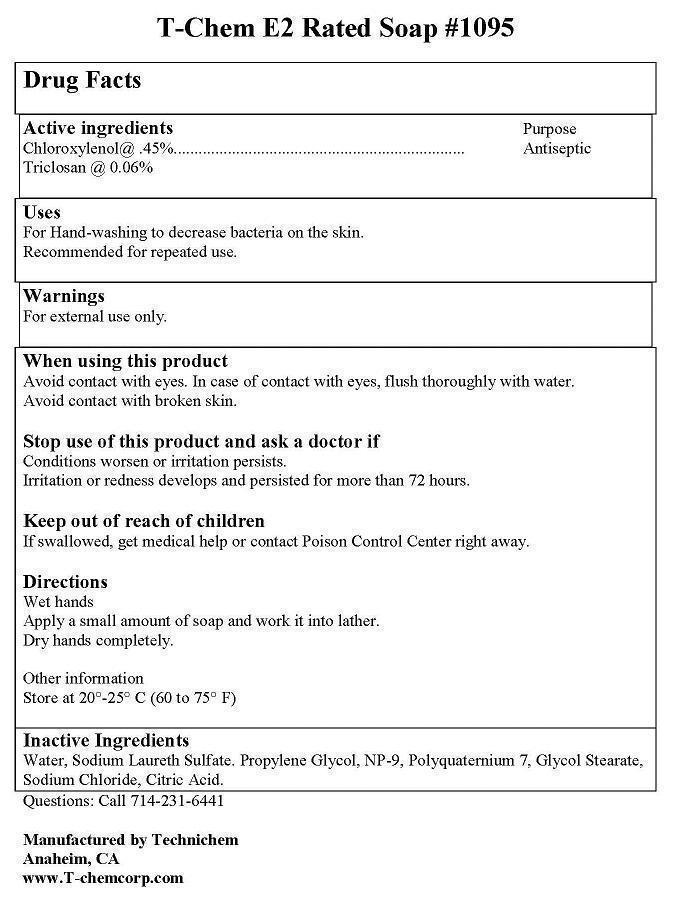
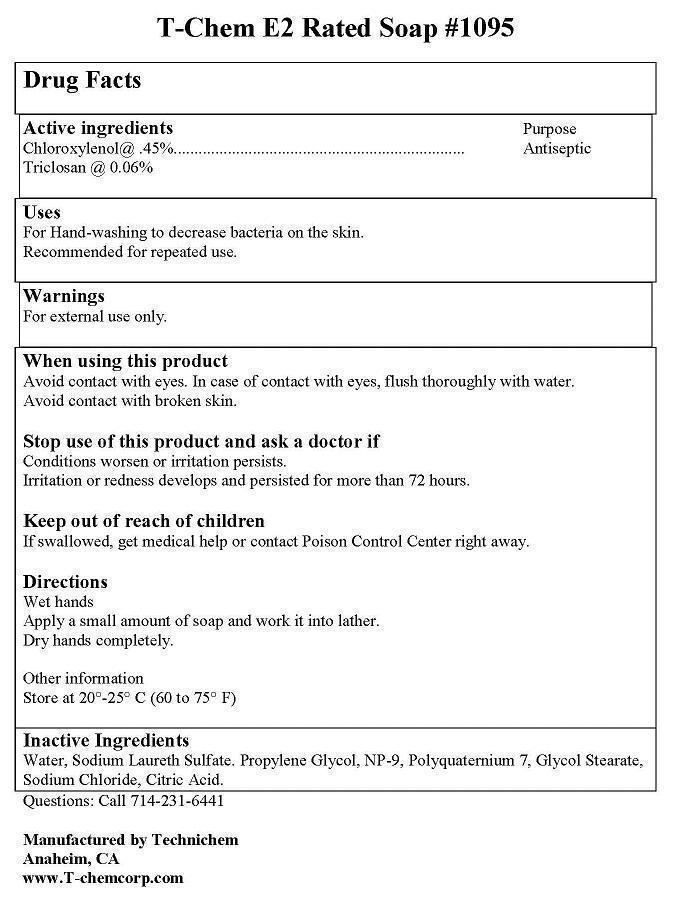
Label: T-CHEM E2 RATED- chloroxylenol, triclosan soap
- NDC Code(s): 50536-192-12
- Packager: AMERICAN CHEMICAL AND SANITARY SUPPLY INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
FIRST AID:
EYES: AVOID CONTACT WITH EYES. IF CONTACT OCCURS, RINSE WITH WATER. CALL PHYSICIAN IF IRRITATION OR REDNESS DEVELOPS AND LASTS.
INGESTION: IN CASE OF ACCIDENTAL INGESTION, GET MEDICAL OR CONTACT A POISON CNTROL CENTER IMMEDIATELY.
CONTAINER DISPOSAL: RINSE THREE TIMES WITH WATER. THEN OFFER FOR RECYCLING OR RECONDITIONING OR PUNCTURE AND DISPOSE OF IN A SANITARY LANDFILL OR BY INCARCERATION OR, IF ALLOWED BY STATE AND LOCAL AAUTHORITIES, BY BURNING. IF BURNED, STAY AWAY FROM SMOKE.
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
T-CHEM E2 RATED
chloroxylenol, triclosan soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50536-192 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.45 g in 100 mL TRICLOSAN (UNII: 4NM5039Y5X) (TRICLOSAN - UNII:4NM5039Y5X) TRICLOSAN 0.06 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM LAURETH SULFATE (UNII: BPV390UAP0) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50536-192-12 800 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 03/12/2014 Labeler - AMERICAN CHEMICAL AND SANITARY SUPPLY INC (177148699)