Label: AQUARELLE- benzalkonium chloride liquid
- NDC Code(s): 70697-804-01
- Packager: INDELPA, S.A DE C.V
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
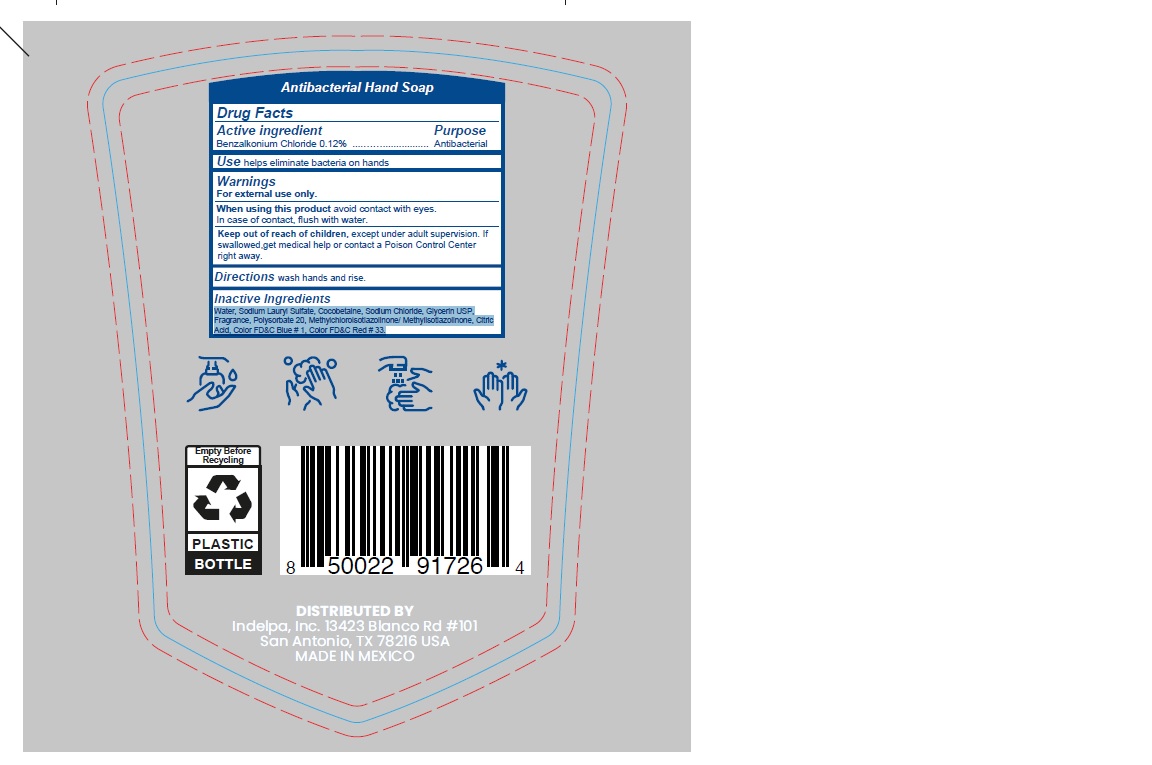
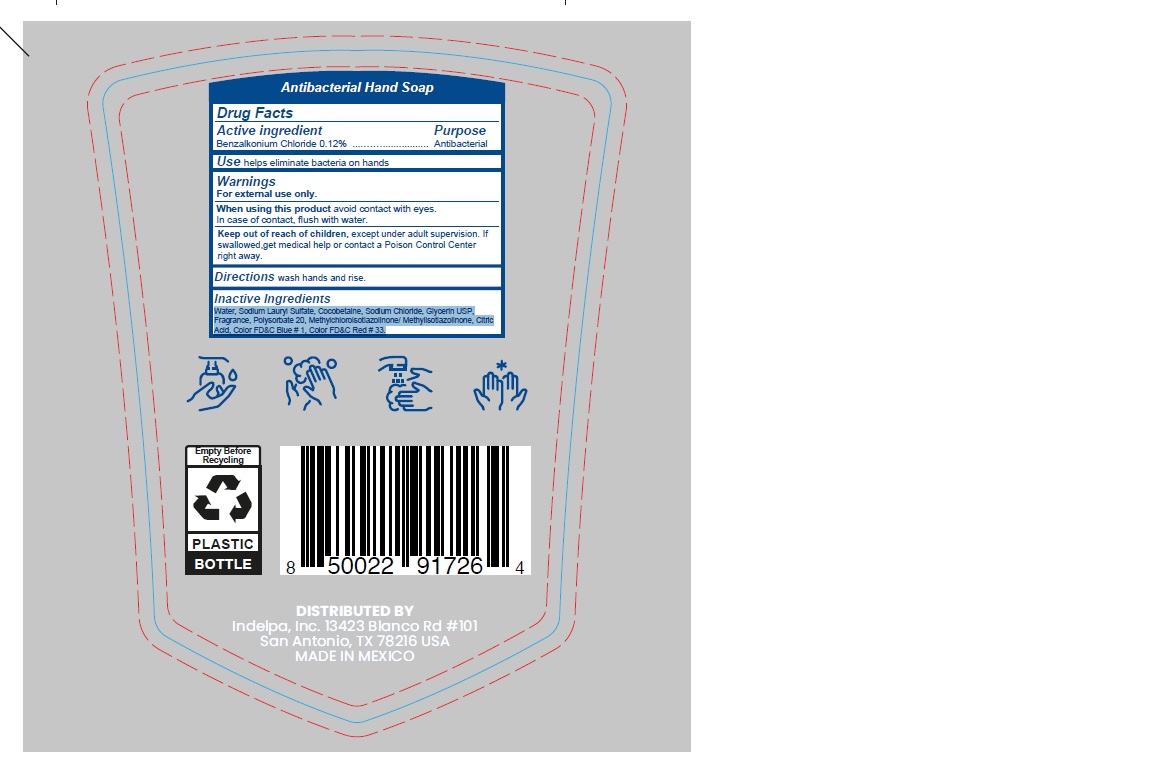
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- INSTRUCTIONS FOR USE
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AQUARELLE
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70697-804 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.12 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM LAURETH SULFATE (UNII: BPV390UAP0) 8.5 mg in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 2.5 mg in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 3 mg in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.0087 mg in 100 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 0.3 mg in 100 mL METHYLCHLOROISOTHIAZOLINONE/METHYLISOTHIAZOLINONE MIXTURE (UNII: 15O9QS218W) 0.1 mg in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 3 mg in 100 mL D&C RED NO. 33 (UNII: 9DBA0SBB0L) 0.000008 mg in 100 mL FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.000035 mg in 100 mL WATER (UNII: 059QF0KO0R) 82.121 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70697-804-01 225 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/11/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/10/2020 Labeler - INDELPA, S.A DE C.V (811072487) Registrant - INDELPA, S.A DE C.V (811072487) Establishment Name Address ID/FEI Business Operations INDELPA, S.A DE C.V 811072487 analysis(70697-804) , manufacture(70697-804) , label(70697-804) , pack(70697-804)

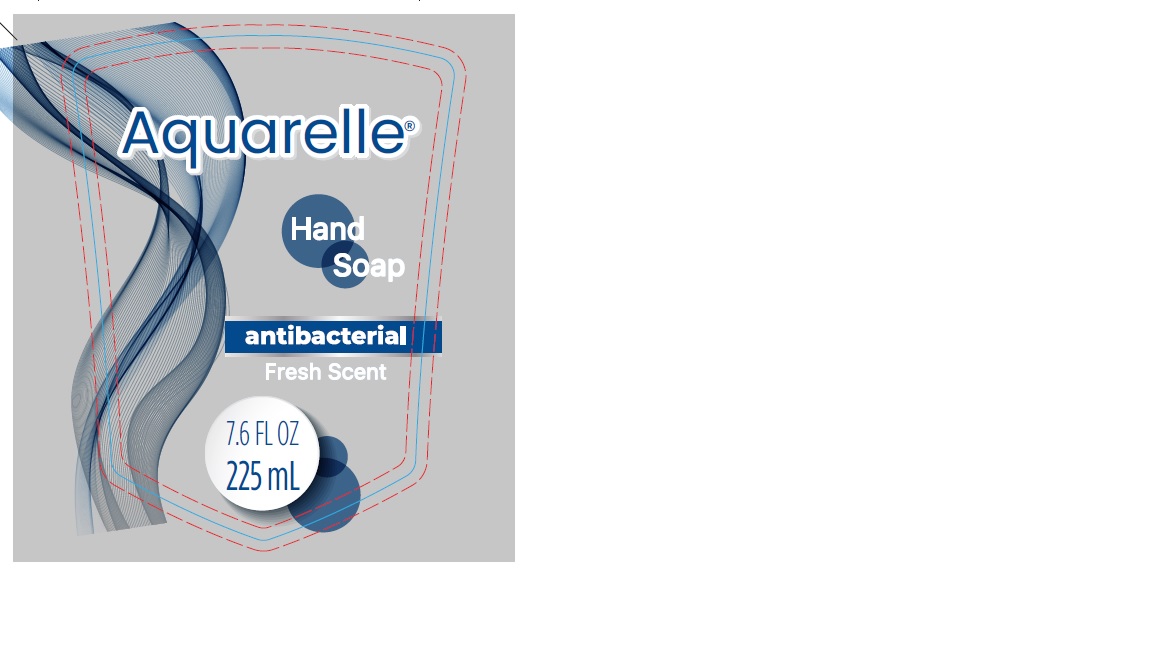
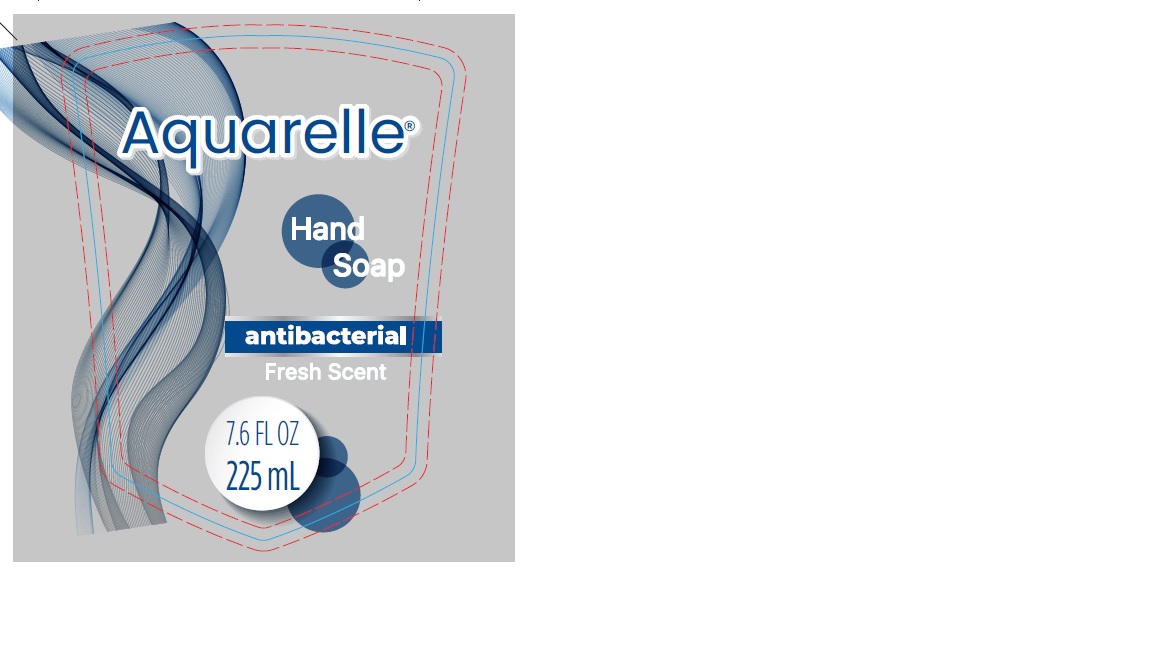 Hand Soap Antibacterial Fresh Scent
Hand Soap Antibacterial Fresh Scent