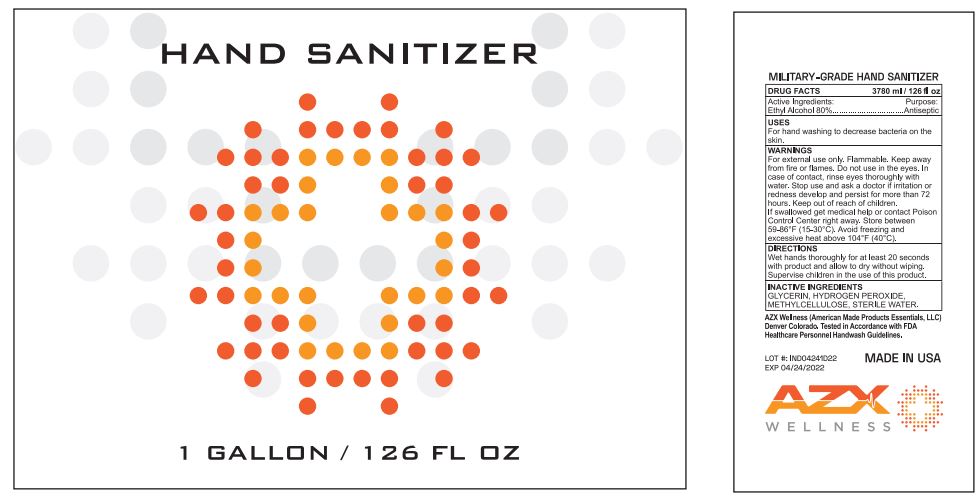
Label: AZX WELLNESS HAND SANITIZER- alcohol gel
- NDC Code(s): 73145-007-01
- Packager: ASC Marketing LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- Active Ingredients:
- USES
- WARNINGS
- DIRECTIONS
- INACTIVE INGREDIENTS
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
AZX WELLNESS HAND SANITIZER
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73145-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.8 mL in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROGEN PEROXIDE (UNII: BBX060AN9V) METHYLCELLULOSE, UNSPECIFIED (UNII: Z944H5SN0H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73145-007-01 3780 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 11/21/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 11/21/2020 Labeler - ASC Marketing LTD (117025198)