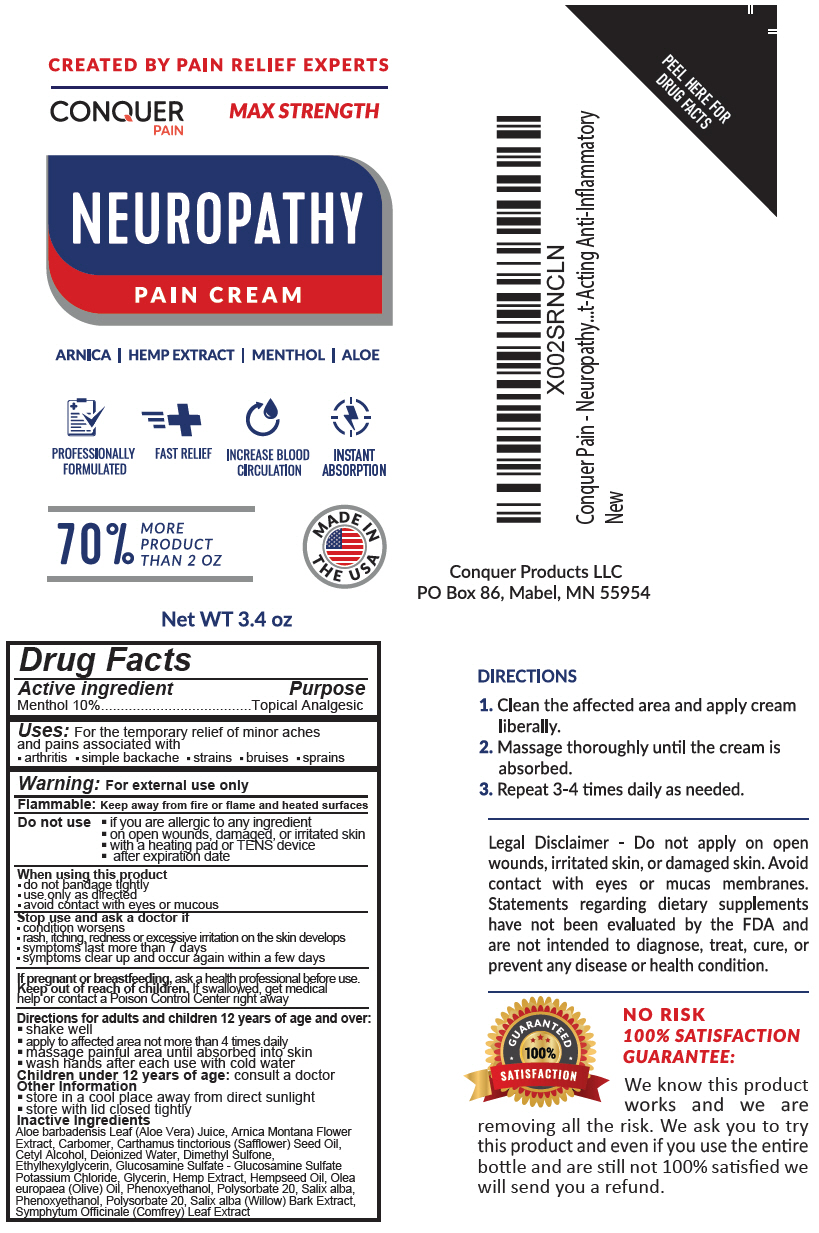
Label: CONQUER PAIN RELIEF CREAM- menthol, unspecified form lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 63374-034-01 - Packager: Steuart Contract Packaging
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warning
For external use only
Flammable: Keep away from fire or flame and heated surfaces
Do not use
- if you are allergic to any ingredient
- on open wounds, damaged, or irritated skin
- with a heating pad or TENS device
- after expiration date
When using this product
- do not bandage tightly
- use only as directed
- avoid contact with eyes or mucous
- Directions for adults and children 12 years of age and over
- Other Information
-
Inactive Ingredients
Aloe barbadensis Leaf (Aloe Vera) Juice, Arnica Montana Flower Extract, Carbomer, Carthamus tinctorious (Safflower) Seed Oil, Cetyl Alcohol, Deionized Water, Dimethyl Sulfone, Ethylhexylglycerin, Glucosamine Sulfate - Glucosamine Sulfate Potassium Chloride, Glycerin, Hemp Extract, Hempseed Oil, Olea europaea (Olive) Oil, Phenoxyethanol, Polysorbate 20, Salix alba, Phenoxyethanol, Polysorbate 20, Salix alba (Willow) Bark Extract, Symphytum Officinale (Comfrey) Leaf Extract
- PRINCIPAL DISPLAY PANEL - 3.4 oz Bottle Label
-
INGREDIENTS AND APPEARANCE
CONQUER PAIN RELIEF CREAM
menthol, unspecified form lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63374-034 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 1.928 g in 96 g Inactive Ingredients Ingredient Name Strength SALIX ALBA BARK (UNII: 205MXS71H7) COMFREY LEAF (UNII: DG4F8T839X) SAFFLOWER OIL (UNII: 65UEH262IS) Water (UNII: 059QF0KO0R) OLIVE OIL (UNII: 6UYK2W1W1E) EDETATE SODIUM (UNII: MP1J8420LU) ALOE VERA LEAF (UNII: ZY81Z83H0X) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) PHENOXYETHANOL (UNII: HIE492ZZ3T) TROLAMINE (UNII: 9O3K93S3TK) POLYSORBATE 20 (UNII: 7T1F30V5YH) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) CETYL ALCOHOL (UNII: 936JST6JCN) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63374-034-01 96 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 08/21/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part348 08/14/2022 Labeler - Steuart Contract Packaging (116952121)