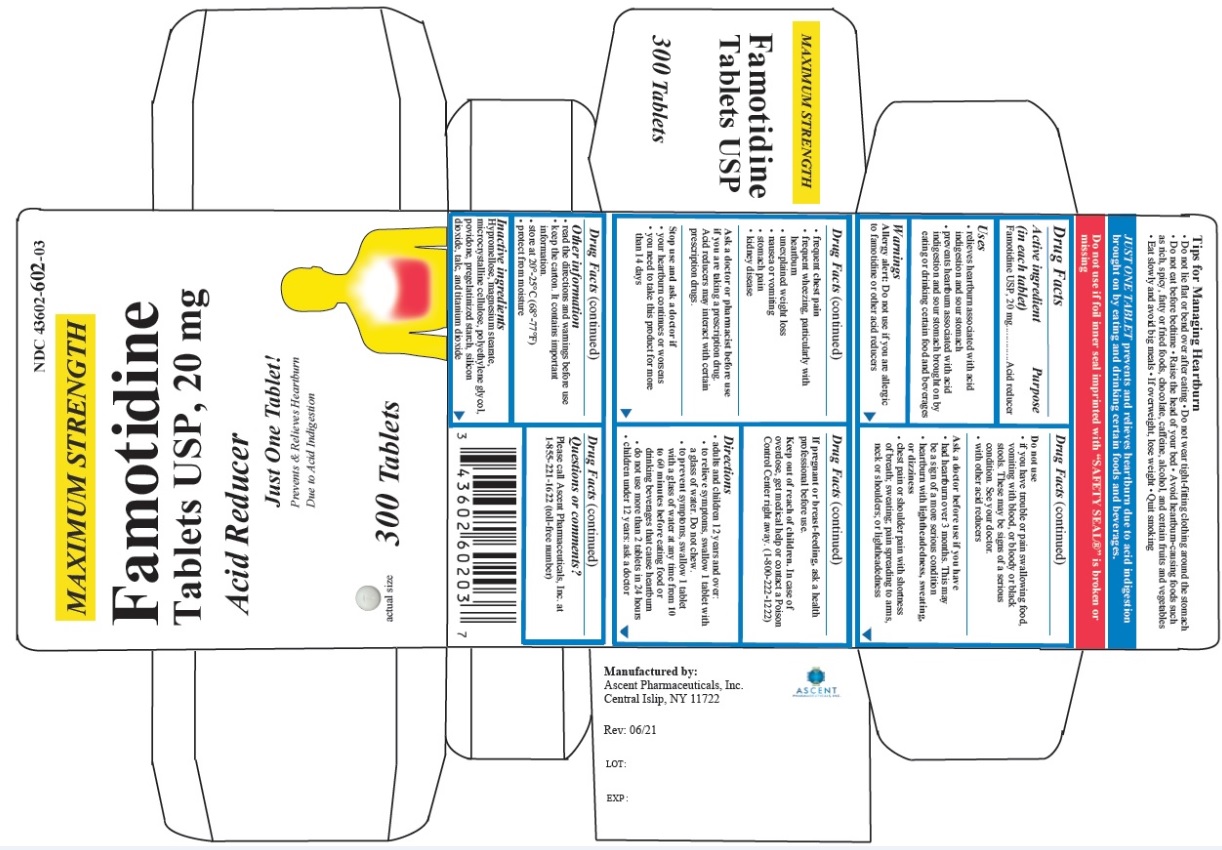
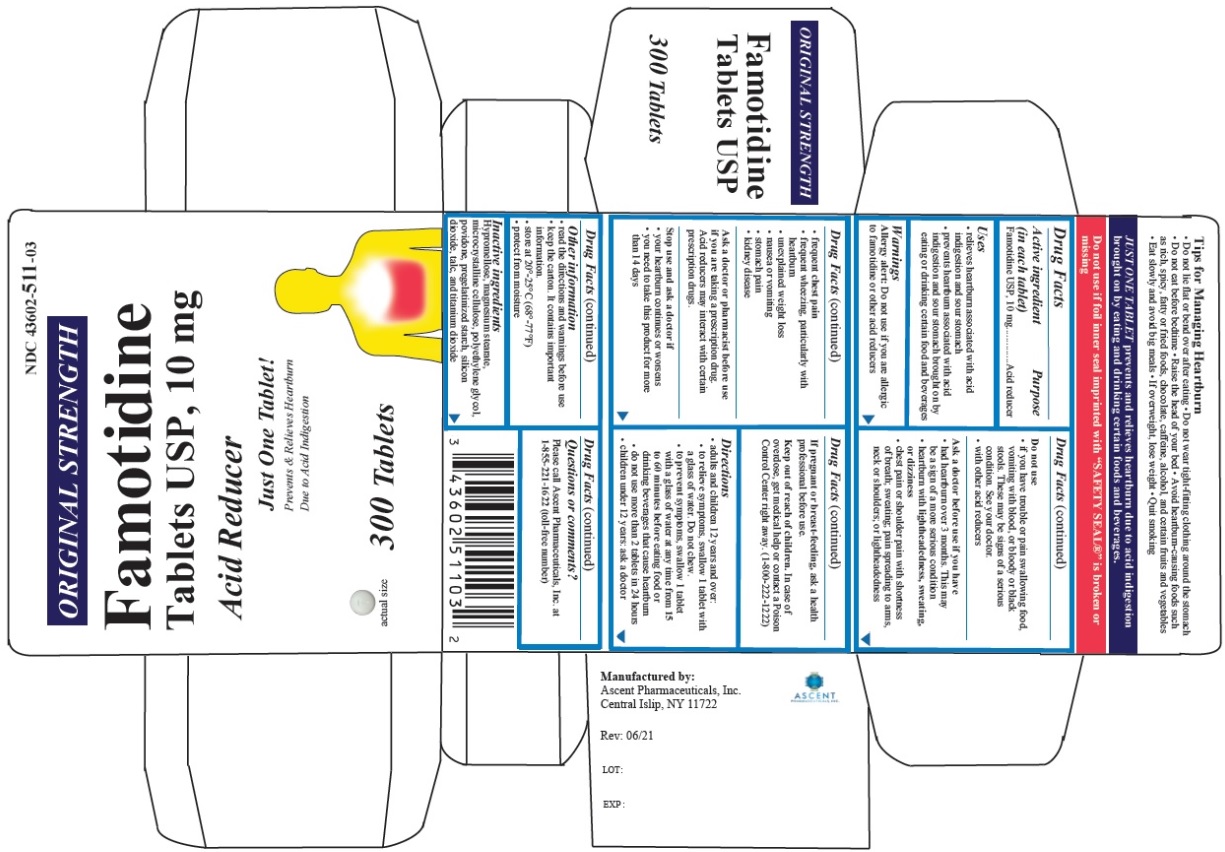
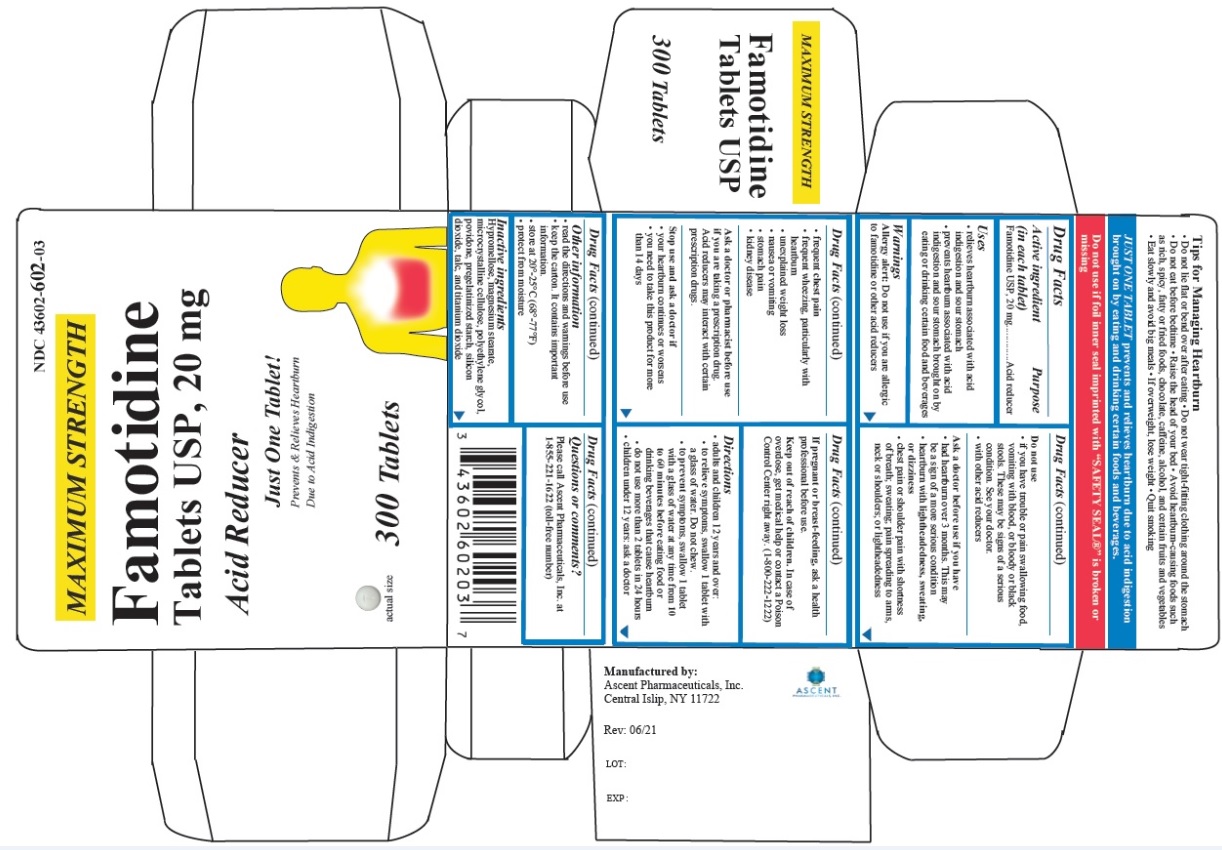
Label: FAMOTIDINE tablet
- NDC Code(s): 43602-511-03, 43602-511-30, 43602-602-03, 43602-602-30
- Packager: Ascent Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
- Warnings
- Do not use
-
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- kidney disease
- ASK DOCTOR/PHARMACIST
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
For Famotidine 10 mg:
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water at any time from 15 to 60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
For Famotidine 20 mg:
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
Tips for Managing Heartburn
- Do not lie flat or bend over after eating
- Do not wear tight-fitting clothing around the stomach
- Do not eat before bedtime
- Raise the head of your bed
- Avoid heartburn-causing foods such as rich, spicy, fatty or fried foods, chocolate, caffeine, alcohol, and certain fruits and vegetables
- Eat slowly and avoid big meals
- If overweight, lose weight
- Quit smoking
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FAMOTIDINE
famotidine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43602-511 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 10 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape ROUND Size 5mm Flavor Imprint Code T;511 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43602-511-30 1 in 1 CARTON 11/03/2021 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:43602-511-03 1 in 1 CARTON 11/03/2021 2 300 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216030 11/03/2021 FAMOTIDINE
famotidine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43602-602 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 20 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code T;602 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43602-602-30 1 in 1 CARTON 11/03/2021 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:43602-602-03 1 in 1 CARTON 11/03/2021 2 300 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216030 11/03/2021 Labeler - Ascent Pharmaceuticals, Inc. (080938961) Establishment Name Address ID/FEI Business Operations Ascent Pharmaceuticals, Inc. 080938961 manufacture(43602-511, 43602-602) , analysis(43602-511, 43602-602) , pack(43602-511, 43602-602)