Label: ANNA GUANCHE MD MIRACLE CREAM THE PIMPLE CREAM- sulfur cream
- NDC Code(s): 80841-100-01
- Packager: Bella Cosmeceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
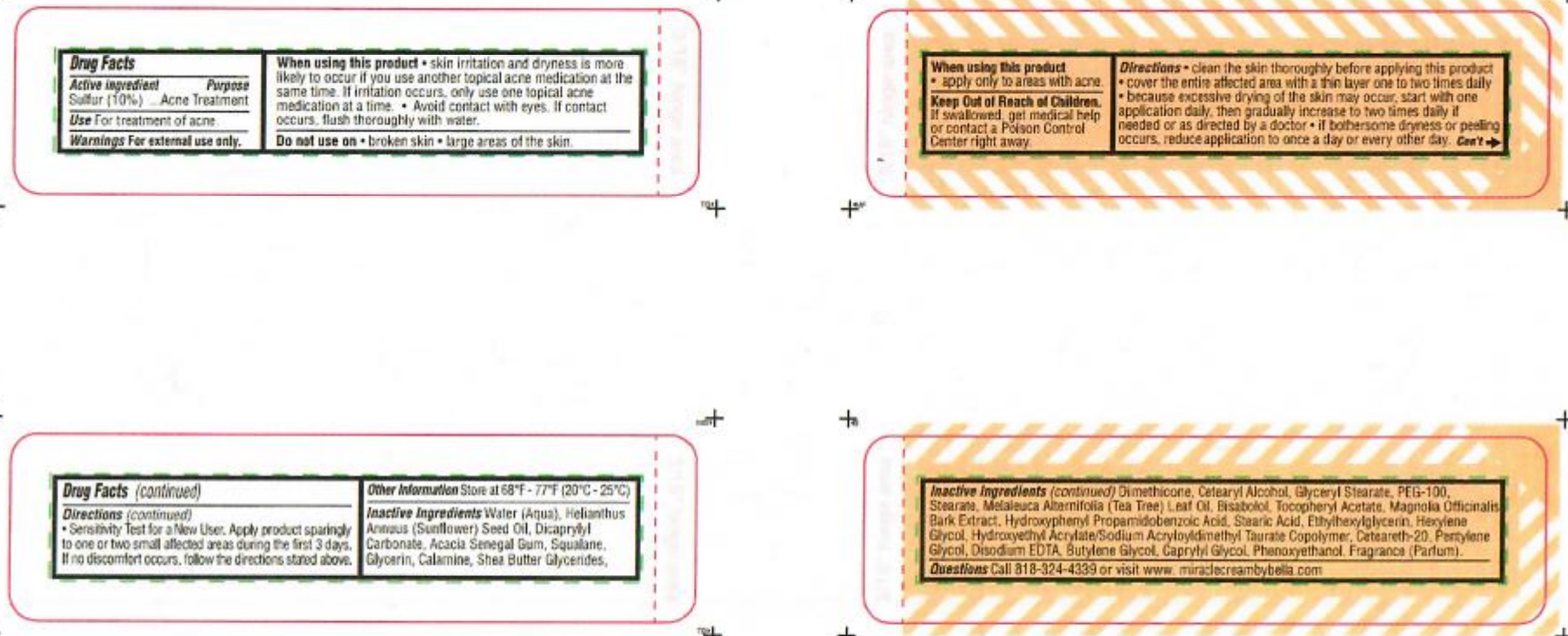
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
-
WHEN USING
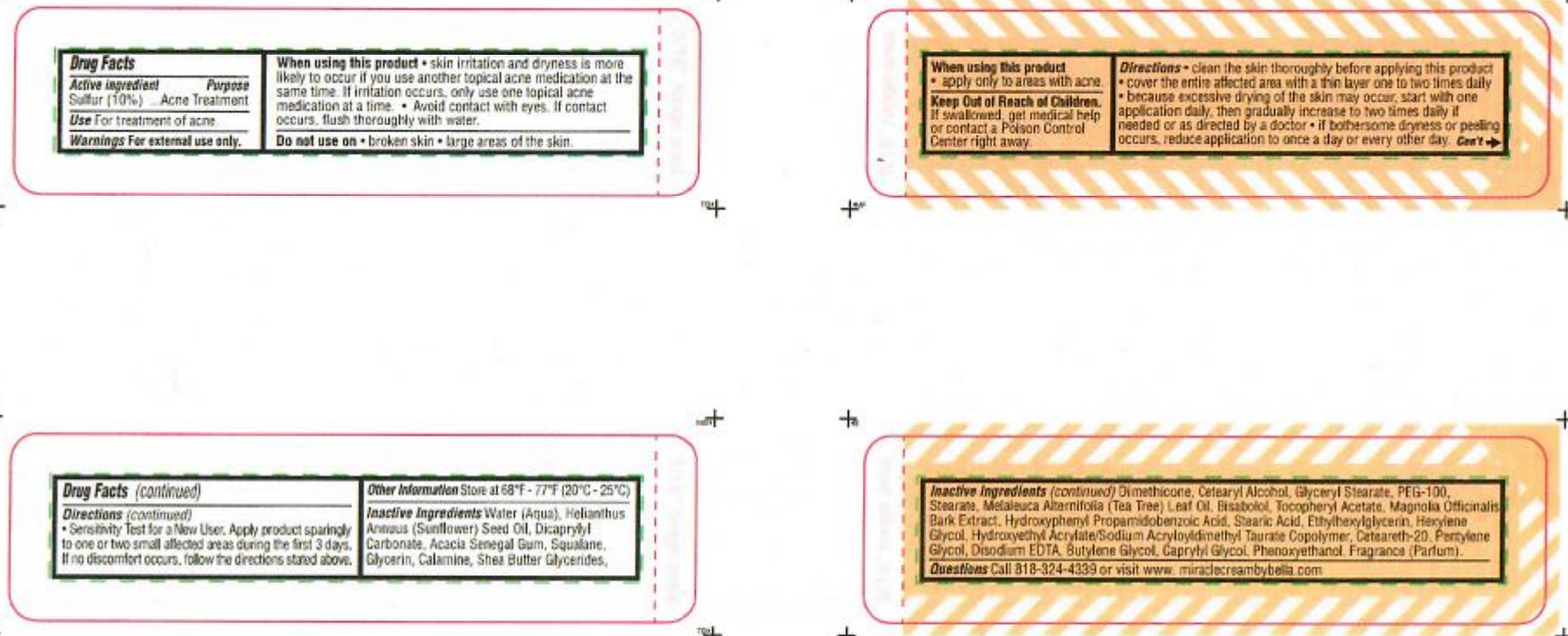
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time.
- if irritation occurs, only use one topical acne medication at a time.
- avoid contact with eyes. If contact occurs, flush thoroughly with water.
- apply only to areas with acne.
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to two times daily
- because excessive drying of the skin may occur, start with one application daily then gradually increase to two times daily if needed
or as directed by a doctor.
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Sensitivity Test for New User. Apply product sparingly to one or two small affected areas during the first 3 days. If no discomforts occurs, follow directions stated above.
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients Water ( Aqua), Helianthus Annuus (Sunflower) Seed Oil, Dicaprylyl Carbonate, Acacia Senegal Gum, Squalane, Glycerin, Calamine, Shea Butter, Glycerides, Dimethicone, Cetearyl Alcohol, Glyceryl Stearate, PEG-100, Stearane, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Bisabolol, Tocopheryl Acetate, Magnolia Officinalis Bark Extract, Hydroxyphenyl Propamidobenzoic Acid, Stearic Acid, Ethylhexylglycerin, Hexylene Glycol, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Ceteareth-20, Pentylene Glycol, Disodium EDTA, Butylene Glycol, Caprylyl Glycol, Penoxyethanol, Fragrance (Parfum)
- DO NOT USE
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANNA GUANCHE MD MIRACLE CREAM THE PIMPLE CREAM
sulfur creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80841-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PENTYLENE GLYCOL (UNII: 50C1307PZG) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) GLYCERIN (UNII: PDC6A3C0OX) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) ISOPROPYL ALCOHOL (UNII: ND2M416302) LONICERA CAPRIFOLIUM FLOWER (UNII: 5N1WD9784U) DIMETHICONE (UNII: 92RU3N3Y1O) PEG-120 GLYCERYL STEARATE (UNII: 6941286E4I) TEA TREE OIL (UNII: VIF565UC2G) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) PEG-100 STEARATE (UNII: YD01N1999R) WATER (UNII: 059QF0KO0R) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) CETYL ALCOHOL (UNII: 936JST6JCN) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) HYDROXYPHENYL PROPAMIDOBENZOIC ACID (UNII: 25KRT26H77) STEARIC ACID (UNII: 4ELV7Z65AP) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) CAPRYLYL GLYCOL (UNII: 00YIU5438U) MAGNOLIA OFFICINALIS BARK (UNII: 5M609NV974) HEXYLENE GLYCOL (UNII: KEH0A3F75J) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SUNFLOWER OIL (UNII: 3W1JG795YI) SQUALANE (UNII: GW89575KF9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80841-100-01 29.5 mL in 1 JAR; Type 0: Not a Combination Product 11/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/17/2020 Labeler - Bella Cosmeceuticals, Inc. (117691855) Registrant - KDC/ONE SOCAL LABORATORIES, LLC (118384240) Establishment Name Address ID/FEI Business Operations KDC/ONE SOCAL LABORATORIES, LLC 118384240 manufacture(80841-100)