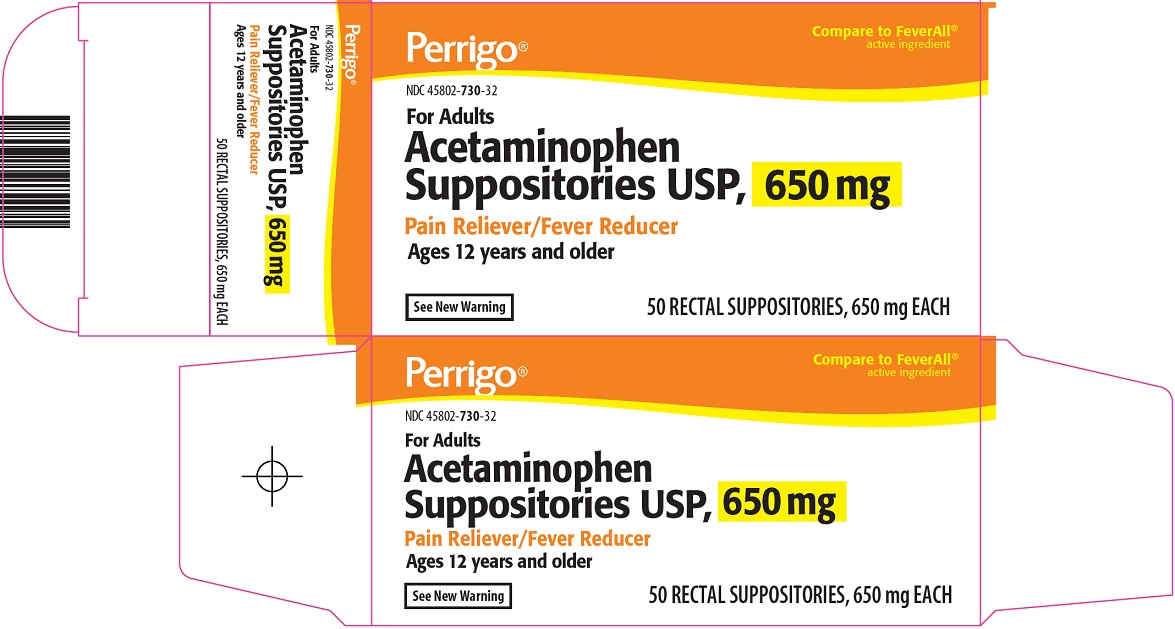
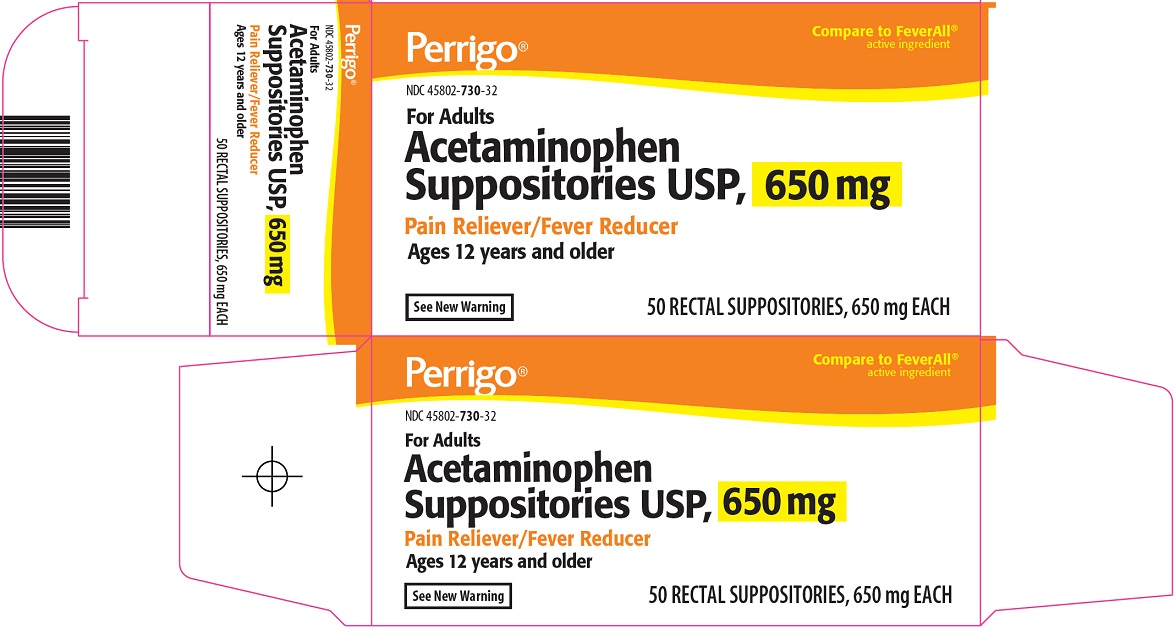
Label: ACETAMINOPHEN PAIN RELIEVER FEVER REDUCER- acetaminophen suppository
- NDC Code(s): 45802-730-00, 45802-730-30, 45802-730-32, 45802-730-33
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 9, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each rectal suppository)
- Purposes
- Uses
-
Warnings
Liver warning:
This product contains acetaminophen. Severe liver damage may occur if
- •
- an adult or child 12 years and older takes more than 6 doses in 24 hours, which is the maximum daily amount
- •
- taken with other drugs containing acetaminophen
- •
- an adult takes 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
For rectal use only
Do not use
- •
- in children under 12 years
- •
- if you are allergic to acetaminophen
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if
- •
- you have liver disease
- •
- you are taking the blood thinning drug warfarin
- Directions
- Other information
- Inactive ingredient
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN PAIN RELIEVER FEVER REDUCER
acetaminophen suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45802-730 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength COTTONSEED OIL (UNII: H3E878020N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-730-32 50 in 1 CARTON 11/22/2010 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:45802-730-30 12 in 1 CARTON 10/28/2010 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:45802-730-33 100 in 1 CARTON 10/28/2010 3 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:45802-730-00 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 10/28/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA070608 10/28/2010 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)