Label: CERAMIDE LIFT AND FIRM EYE SPF 15- oxybenzone, octinoxate emulsion
- NDC Code(s): 10967-667-61
- Packager: REVLON
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
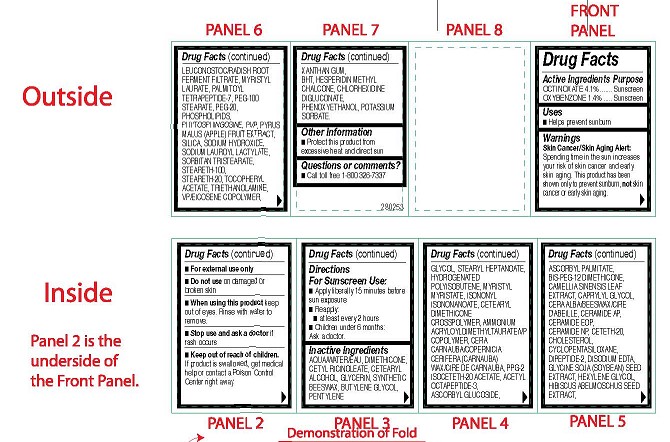
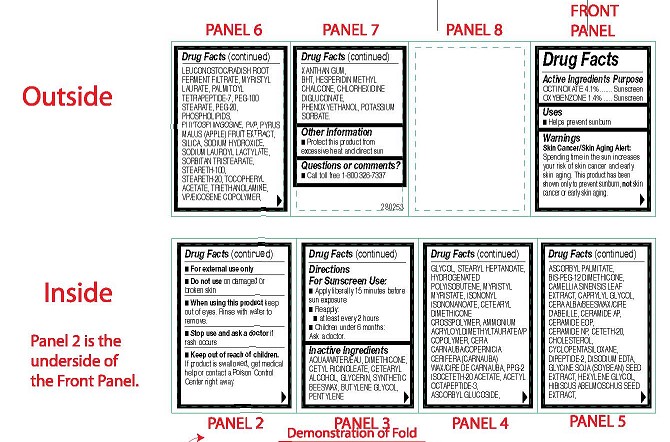
- ACTIVE INGREDIENTS PURPOSE
-
OTHER INGREDIENTS
Other Ingredients: Aqua/Water/Eau, Dimethicone, Cetyl Ricinoleate, Cetearyl Alcohol,
Glycerin, Synthetic Beeswax, Butylene Glycol, Pentylene Glycol, Stearyl Heptanoate,
Hydrogenated Polyisobutene, Myristyl Myristate, Isononyl Isononanoate, Cetearyl Dimethicone
Crosspolymer, Ammonium Acryloyldimethyltaurate/VP Copolymer, Cera Carnauba/Copernicia
Cerifera (Carnauba) Wax/Cire de carnauba, PPG-2 Isoceteth-20 Acetate, Acetyl Octapeptide-3,
Ascorbyl Glucoside, Ascorbyl Palmitate, Bis-PEG-12 Dimethicone, Camellia Sinensis Leaf
Extract, Caprylyl Glycol, Cera Alba/Beeswax/Cire D'abeille, Ceramide AP, Ceramide EOP,
Ceramide NP, Ceteth-20, Cholesterol, Cyclopentasiloxane, Dipeptide-2, Disodium EDTA,
Glycine Soja (Soybean) Seed Extract, Hexylene Glycol, Hibiscus Abelmoschus Seed Extract,
Leuconostoc/Radish Root Ferment Filtrate, Myristyl Laurate, Palmitoyl Tetrapeptide-7, PEG-100
Stearate, PEG-20, Phospholipids, Phytosphingosine, PVP, Pyrus Malus (Apple) Fruit Extract,
Silica, Sodium Hydroxide, Sodium Lauroyl Lactylate, Sorbitan Tristearate, Steareth-100,
Steareth-20, Tocopheryl Acetate, Triethanolamine, VP/Eicosene Copolymer, Xanthan Gum,
BHT, Hesperidin Methyl Chalcone, Chlorhexidine Digluconate, Phenoxyethanol, Potassium
Sorbate. - USES
-
WARNINGS
Warnings
Skin Cancer/Skin Aging Alert:
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.- For external use only
- Do not use on damaged or broken skin
- When using this product keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash occurs
- Keep out of reach of children.
If product is swallowed, get medical
help or contact a Poison Control
Center right away. - DIRECTIONS FOR SUNSCREEN USE
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- DRUG FACTS BOX
- CARTON ART
-
INGREDIENTS AND APPEARANCE
CERAMIDE LIFT AND FIRM EYE SPF 15
oxybenzone, octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-667 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 4.1 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 1.4 mg in 1 g Inactive Ingredients Ingredient Name Strength SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) ACETYL OCTAPEPTIDE-3 (UNII: 8K14HJF88S) GREEN TEA LEAF (UNII: W2ZU1RY8B0) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CERAMIDE NP (UNII: 4370DF050B) DIMETHICONE (UNII: 92RU3N3Y1O) PPG-2 ISOCETETH-20 ACETATE (UNII: BI6C7YO419) SOYBEAN (UNII: L7HT8F1ZOD) PENTYLENE GLYCOL (UNII: 50C1307PZG) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) ABELMOSCHUS MOSCHATUS SEED (UNII: UN2QZ55I88) BIS-PEG-12 DIMETHICONE (500 MPA.S) (UNII: 2CNS542YRT) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) WATER (UNII: 059QF0KO0R) STEARETH-20 (UNII: L0Q8IK9E08) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) STEARYL HEPTANOATE (UNII: 2M4UGL1NCN) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) XANTHAN GUM (UNII: TTV12P4NEE) STEARETH-100 (UNII: 4OH5W9UM87) TROLAMINE (UNII: 9O3K93S3TK) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SORBITAN TRISTEARATE (UNII: 6LUM696811) PEG-100 STEARATE (UNII: YD01N1999R) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) CETYL RICINOLEATE (UNII: 1P677500YD) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) CETETH-20 (UNII: I835H2IHHX) ASCORBYL PALMITATE (UNII: QN83US2B0N) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) MYRISTYL LAURATE (UNII: 58U0NZN2BT) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) CARNAUBA WAX (UNII: R12CBM0EIZ) POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) VALYLTRYPTOPHAN (UNII: 3G64B4AFQN) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) APPLE (UNII: B423VGH5S9) CHOLESTEROL (UNII: 97C5T2UQ7J) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-667-61 14.4 g in 1 JAR; Type 0: Not a Combination Product 04/19/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/19/2019 Labeler - REVLON (788820165) Establishment Name Address ID/FEI Business Operations Cosmetic Essence, LLC dba Voyant Beauty 032565959 manufacture(10967-667)