Label: CERAMIDE LIFT AND FIRM- octinoxate, oxybenzone, avobenzone, octisalate, octocrylene emulsion
- NDC Code(s): 10967-666-17
- Packager: REVLON
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- USES
-
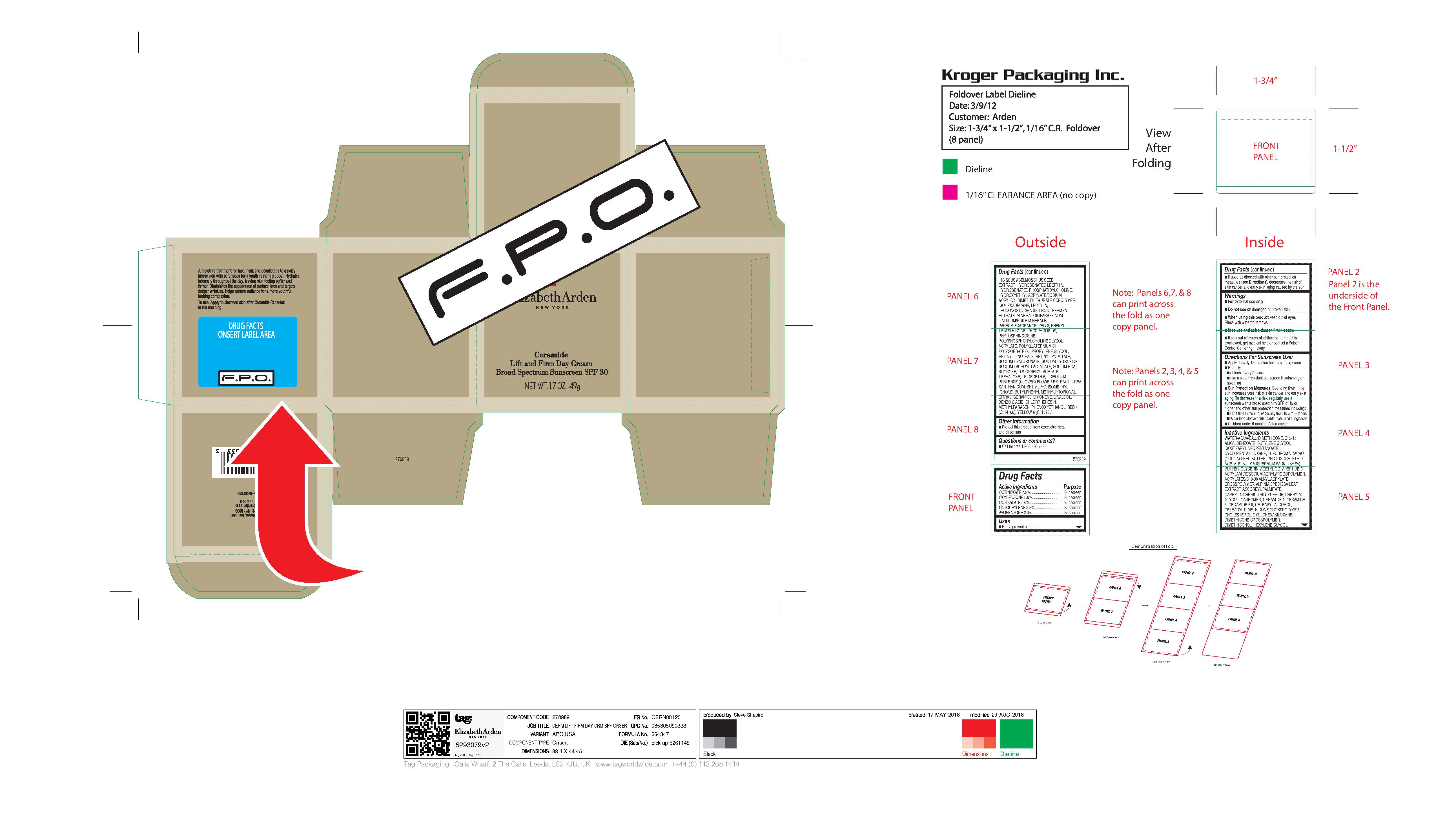
WARNINGS
Warnings
■ For external use only
■ Do not use on damaged or broken skin
■ When using this product keep out of eyes. Rinse with water to remove.
■ Stop use and ask a doctor if rash occurs
■ Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away. -
DIRECTIONS FOR SUNSCREEN USE
Directions For Sunscreen Use:
■ Apply liberally 15 minutes before sun exposure
■ Reapply:
■ at least every 2 hours
■ use a water resistant sunscreen if swimming or sweating
■ Sun Protection Measures. Spending time in the
sun increases your risk of skin cancer and early skin
aging. To decrease this risk, regularly use a
sunscreen with a broad spectrum SPF of 15 or
higher and other sun protection measures including:
■ Limit time in the sun, especially from 10 a.m. – 2 p.m.
■ Wear long-sleeve shirts, pants, hats, and sunglasses
■ Children under 6 months: Ask a doctor. -
OTHER INGREDIENTS
WATER/AQUA/EAU, DIMETHICONE, C12-15 ALKYL BENZOATE, BUTYLENE GLYCOL, ISOSTEARYL NEOPENTANOATE, CYCLOPENTASILOXANE, THEOBROMA CACAO (COCOA) SEED BUTTER, PPG-2 ISOCETETH-20 ACETATE, BUTYROSPERMUM PARKII (SHEA) BUTTER, GLYCERIN, ACETYL OCTAPEPTIDE-3, ACRYLAMIDE/SODIUM ACRYLATE COPOLYMER, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, ALPINIA SPECIOSA LEAF EXTRACT, ASCORBYL PALMITATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, CAPRYLYL GLYCOL, CARBOMER, CERAMIDE 1, CERAMIDE 3, CERAMIDE 6 II, CETEARYL ALCOHOL, CETEARYL DIMETHICONE CROSSPOLYMER, CHOLESTEROL, CYCLOHEXASILOXANE, DIMETHICONE CROSSPOLYMER, DIMETHICONOL, HEXYLENE GLYCOL, HIBISCUS ABELMOSCHUS SEED EXTRACT, HYDROGENATED LECITHIN, HYDROGENATED PHOSPHATIDYLCHOLINE, HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, ISOHEXADECANE, LECITHIN, LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE, MINERAL OIL/PARAFFINUM LIQUIDUM/HUILE MINERALE, PARFUM/FRAGRANCE, PEG-8, PHENYL TRIMETHICONE, PHOSPHOLIPIDS, PHYTOSPHINGOSINE, POLYPHOSPHORYLCHOLINE GLYCOL ACRYLATE, POLYQUATERNIUM-51, POLYSORBATE 60, PROPYLENE GLYCOL, RETINYL LINOLEATE, RETINYL PALMITATE, SODIUM HYALURONATE, SODIUM HYDROXIDE, SODIUM LAUROYL LACTYLATE, SODIUM PCA, SUCROSE, TOCOPHERYL ACETATE, TREHALOSE, TRIDECETH-6, TRIFOLIUM PRATENSE (CLOVER) FLOWER EXTRACT, UREA, XANTHAN GUM, BHT, ALPHA-ISOMETHYL IONONE, BUTYLPHENYL METHYLPROPIONAL, CITRAL, GERANIOL, LIMONENE, LINALOOL, BENZOIC ACID, CHLORPHENESIN, METHYLPARABEN, PHENOXYETHANOL, RED 4 (CI 14700), YELLOW 6 (CI 15985).
- DOSAGE & ADMINISTRATION
- WARNINGS
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- DRUG FACTS BOX
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CERAMIDE LIFT AND FIRM
octinoxate, oxybenzone, avobenzone, octisalate, octocrylene emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10967-666 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 5 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.2 mg in 1 g Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) ACETYL OCTAPEPTIDE-3 (UNII: 8K14HJF88S) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) CERAMIDE 6 II (UNII: F1X8L2B00J) CERAMIDE 3 (UNII: 4370DF050B) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) COCOA BUTTER (UNII: 512OYT1CRR) PPG-2 ISOCETETH-20 ACETATE (UNII: BI6C7YO419) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) CITRAL (UNII: T7EU0O9VPP) CERAMIDE 1 (UNII: 5THT33P7X7) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) CHOLESTEROL (UNII: 97C5T2UQ7J) ASCORBYL PALMITATE (UNII: QN83US2B0N) XANTHAN GUM (UNII: TTV12P4NEE) METHYLPARABEN (UNII: A2I8C7HI9T) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) GLYCERIN (UNII: PDC6A3C0OX) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ABELMOSCHUS MOSCHATUS SEED (UNII: UN2QZ55I88) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MINERAL OIL (UNII: T5L8T28FGP) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) LIMONENE, (+)- (UNII: GFD7C86Q1W) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) ALPINIA ZERUMBET LEAF (UNII: MS8P33AMKX) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) ISOHEXADECANE (UNII: 918X1OUF1E) UREA (UNII: 8W8T17847W) PHENOXYETHANOL (UNII: HIE492ZZ3T) RETINYL LINOLEATE (UNII: 61911N8D6W) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM HYDROXIDE (UNII: 55X04QC32I) BENZOIC ACID (UNII: 8SKN0B0MIM) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) LINALOOL, (+/-)- (UNII: D81QY6I88E) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 4:1) (UNII: X8Q92E1CPM) POLYSORBATE 60 (UNII: CAL22UVI4M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GERANIOL (UNII: L837108USY) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) TREHALOSE (UNII: B8WCK70T7I) FD&C RED NO. 4 (UNII: X3W0AM1JLX) CHLORPHENESIN (UNII: I670DAL4SZ) TRIDECETH-6 (UNII: 3T5PCR2H0C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10967-666-17 49 g in 1 JAR; Type 0: Not a Combination Product 10/24/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/24/2016 Labeler - REVLON (788820165) Establishment Name Address ID/FEI Business Operations Cosmetic Essence, LLC dba Voyant Beauty 032565959 manufacture(10967-666)