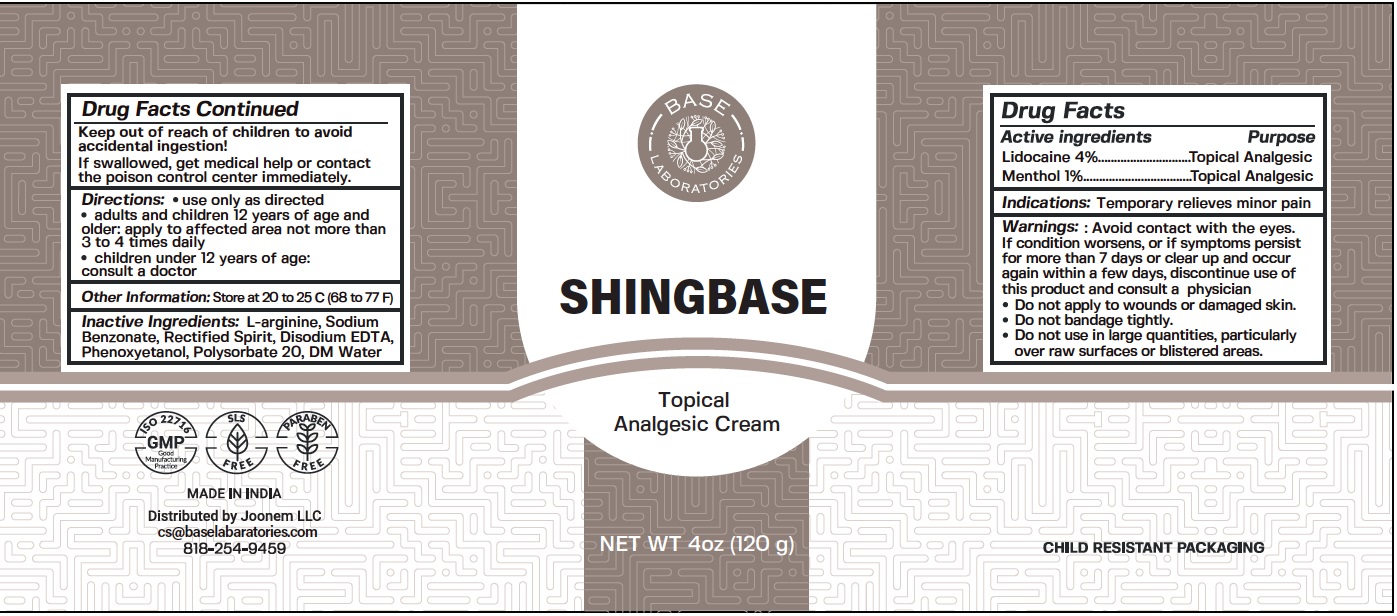
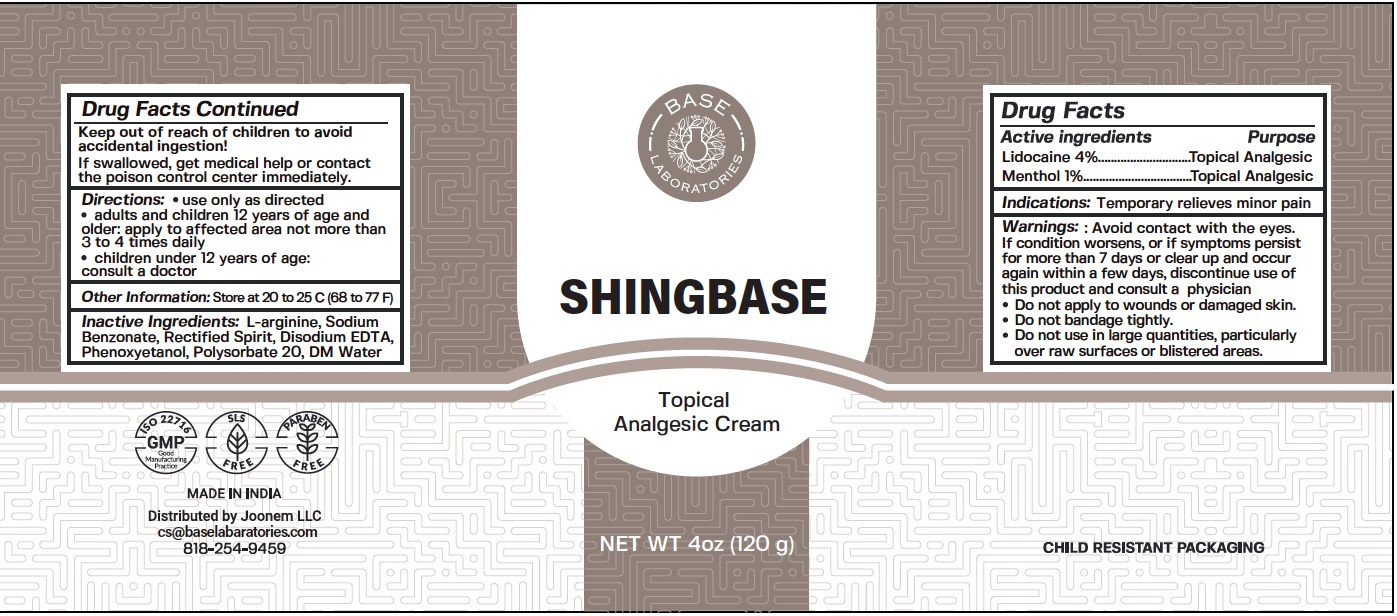
Label: SHINGBASE TOPICAL ANALGESIC- lidocaine, menthol cream
- NDC Code(s): 80327-002-01
- Packager: JOONEM LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Indications:
- Warnings:
- Directions:
- Other Information:
- Inactive Ingredients:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SHINGBASE TOPICAL ANALGESIC
lidocaine, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80327-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 40 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 mg in 1 g Inactive Ingredients Ingredient Name Strength ARGININE (UNII: 94ZLA3W45F) SODIUM BENZOATE (UNII: OJ245FE5EU) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80327-002-01 120 g in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/01/2020 Labeler - JOONEM LLC (117633878)