Label: GAS RELIEF DROPS- dimethicone liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 70570-0197-1 - Packager: Arymar Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 4, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
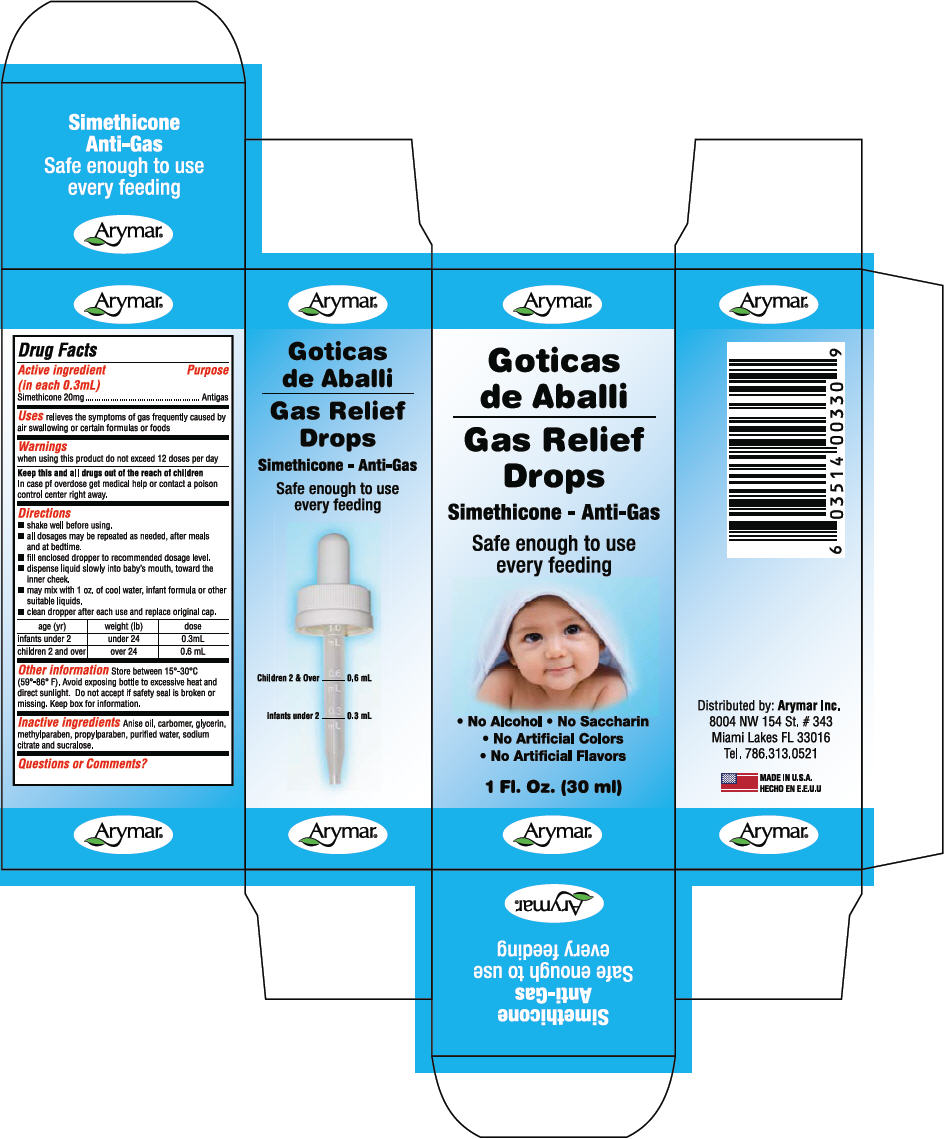
- Active ingredient (in each 0.3mL)
- Purpose
- Uses
- Warnings
-
Directions
- shake well before using.
- all dosages may be repeated as needed, after meals and at bedtime.
- fill enclosed dropper to recommended dosage level.
- dispense liquid slowly into baby's mouth, toward the inner cheek.
- may mix with 1 oz. of cool water, infant formula or other suitable liquids.
- clean dropper after each use and replace original cap.
age (yr) weight (lb) dose infants under 2 under 24 0.3mL children 2 and over over 24 0.6 mL - Other information
- Inactive ingredients
- Questions or Comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
GAS RELIEF DROPS
dimethicone liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70570-0197 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dimethicone (UNII: 92RU3N3Y1O) (Dimethicone - UNII:92RU3N3Y1O) Dimethicone 20 mg in 0.3 mL Inactive Ingredients Ingredient Name Strength Anise Oil (UNII: 6Y89129C8H) Carboxypolymethylene (UNII: 0A5MM307FC) Glycerin (UNII: PDC6A3C0OX) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Water (UNII: 059QF0KO0R) Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) Sucralose (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70570-0197-1 1 in 1 CARTON 02/01/2015 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part332 02/01/2015 Labeler - Arymar Inc. (021650273)