Label: SALINE- nasal spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 53943-704-15, 53943-704-30 - Packager: DISCOUNT DRUG MART, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 25, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients
- Questions or comments?
-
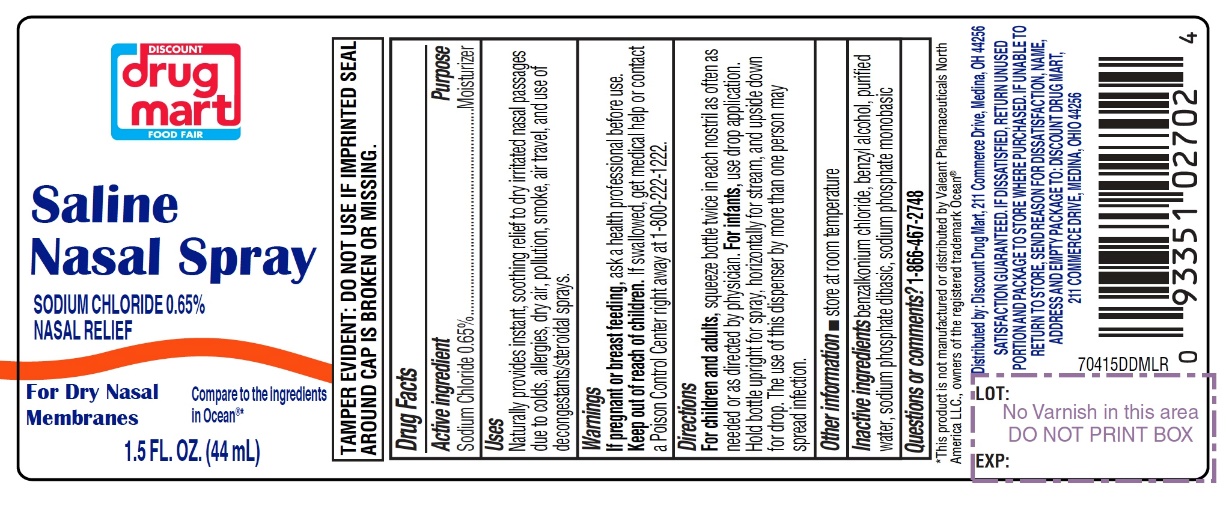
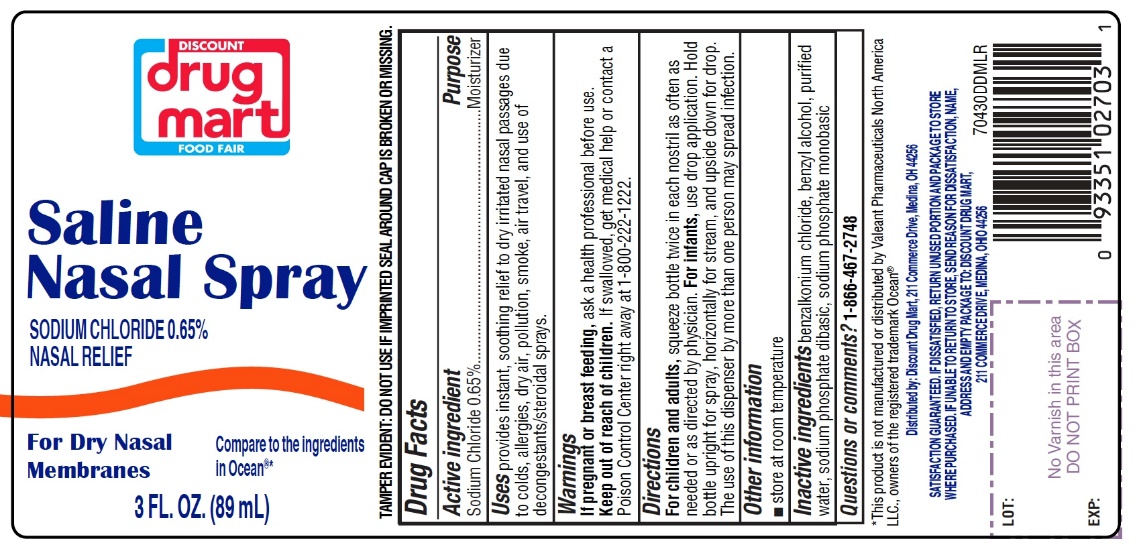
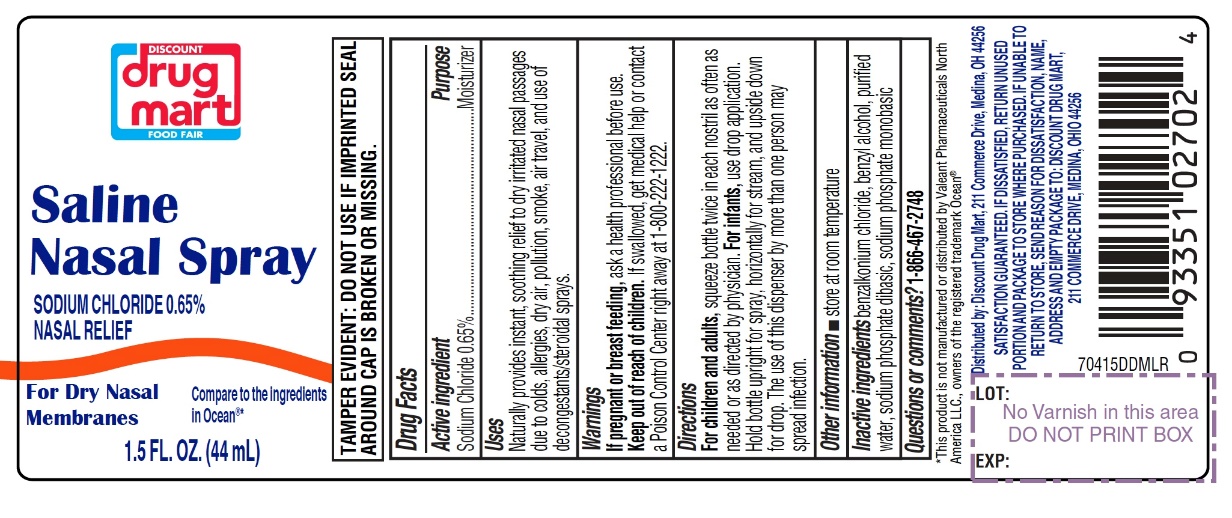
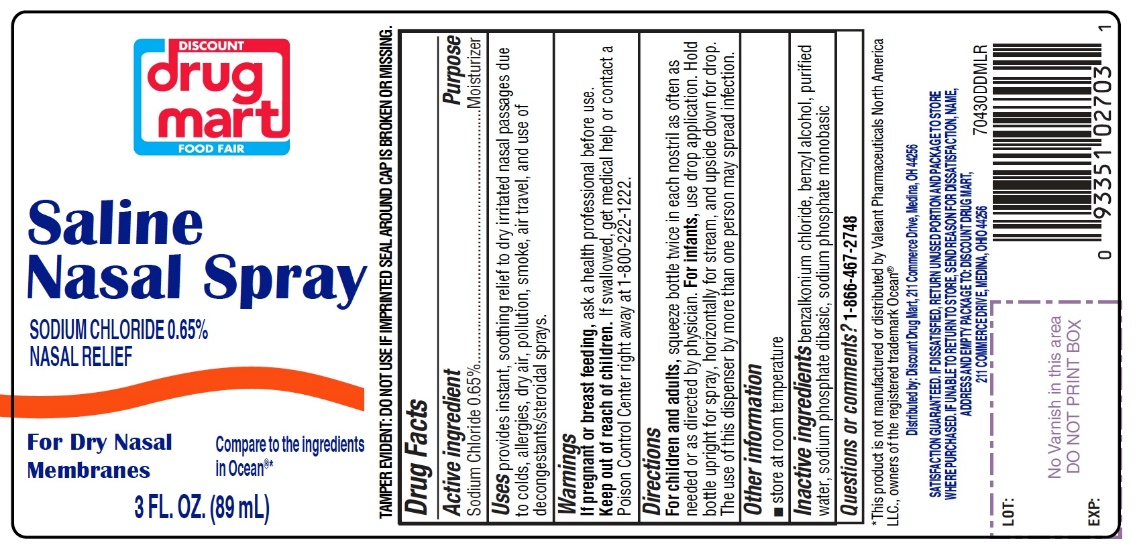
Saline Nasal Spray
Compare to the active ingredient in Ocean®*
Saline Nasal Spray
Sodium Chloride 0.65%
- •
- Instantly relieves dry nasal passages caused by sinus, cold and allergy medications and dry air
- •
- Safe for frequent daily use
- •
- Gentle enough for infants
- •
- Natural, non-medicated relief for stuffy noses
100% SATISFACTION GUARANTEED OR YOUR MONEY BACK
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SEAL AROUND CAP IS BROKEN OR MISSING.
Distributed by:
Package label for 44 mL
-
INGREDIENTS AND APPEARANCE
SALINE
nasal sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53943-704 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 6.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53943-704-15 1 in 1 CARTON 09/30/2020 1 44 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:53943-704-30 1 in 1 CARTON 09/30/2020 2 88 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 09/30/2020 Labeler - DISCOUNT DRUG MART, INC. (047741335)