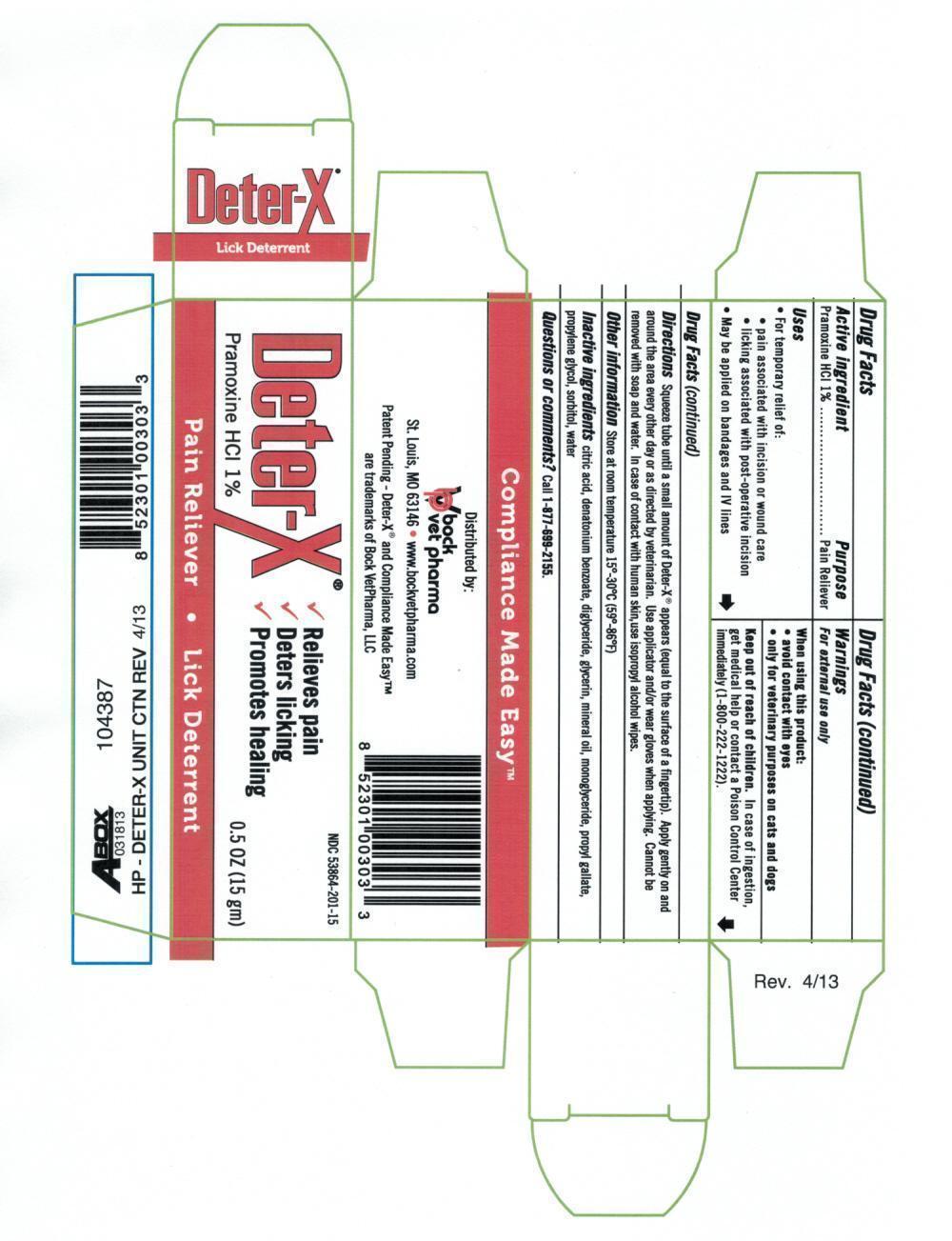
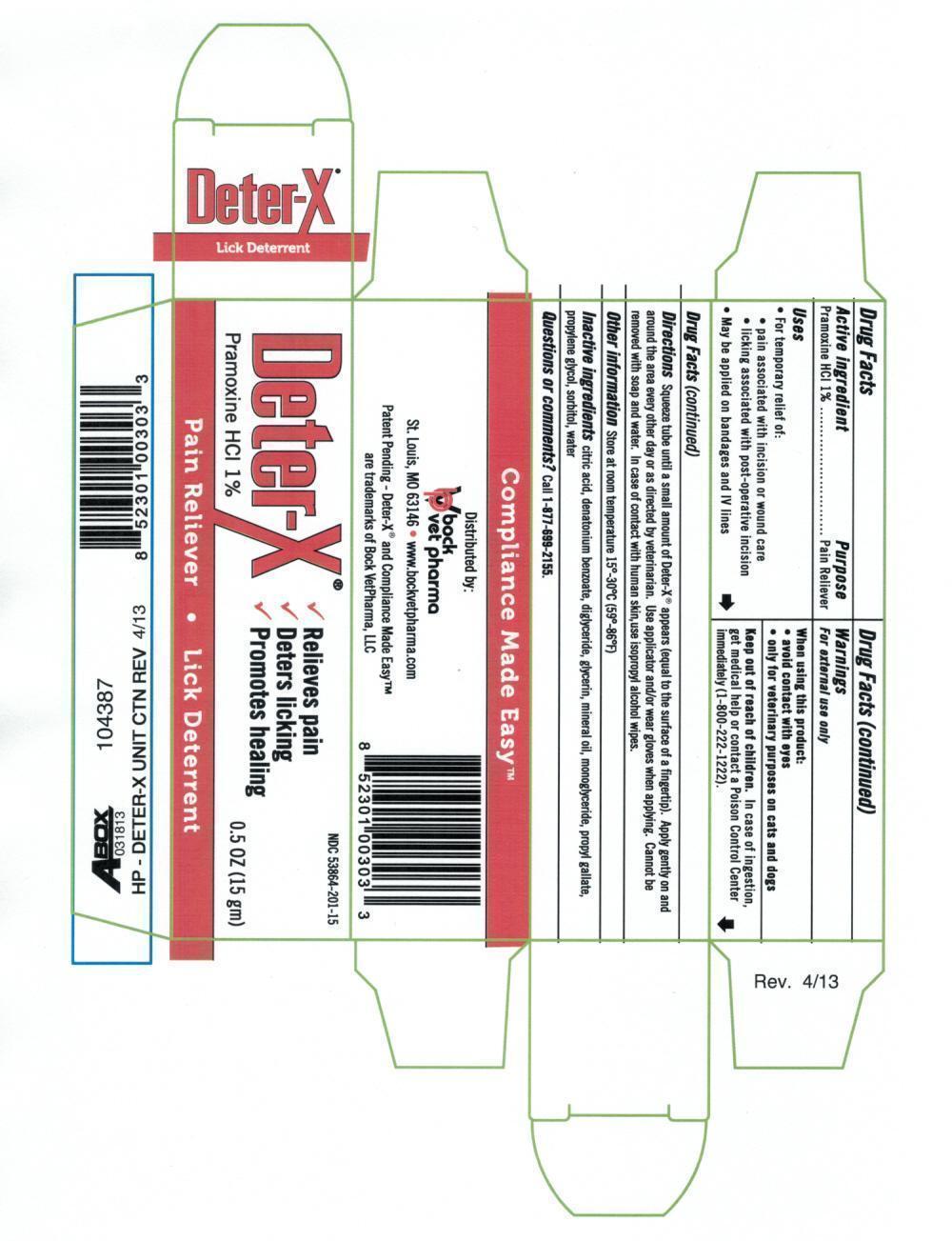
Label: DETER-X- pramoxine hcl ointment
- NDC Code(s): 59228-601-15
- Packager: EMS ACQUISITION CORP.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DETER-X
pramoxine hcl ointmentProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:59228-601 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 0.15 g in 15 g Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) DIGLYCERIN (UNII: 3YC120743U) GLYCERIN (UNII: PDC6A3C0OX) DIACETYLATED MONOGLYCERIDES (UNII: 5Z17386USF) MINERAL OIL (UNII: T5L8T28FGP) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59228-601-15 15 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/02/2013 Labeler - EMS ACQUISITION CORP. (048602791) Establishment Name Address ID/FEI Business Operations EMS ACQUISITION CORP. 048602791 manufacture Establishment Name Address ID/FEI Business Operations AbbVie Inc. 078521674 api manufacture