Label: GILTUSS TOTAL RELEASE- guaifenesin, dextromethorphan hbr, and phenylephrine hcl tablet, film coated
EXACTUSS TOTAL RELEASE- guaifenesin, dextromethorphan hbr, and phenylephrine hcl tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 58552-317-01, 58552-317-02, 58552-327-01 - Packager: Gil Pharmaceutical Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 18, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
-
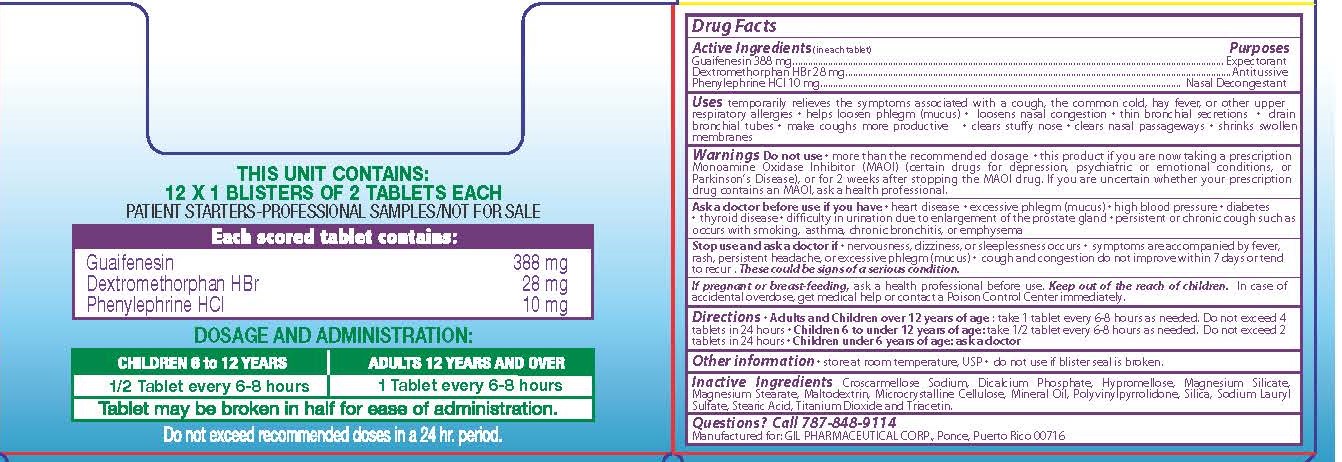
Uses
- temporarily relieves these symptoms associated with a cough, the common cold, hay fever, or other upper respiratory allergies
- helps loosen phlegm (mucus)
- loosens nasal congestion
- thin bronchial secretions
- drain bronchial tubes
- make coughs more productive
- clears stuffy nose
- clear nasal passageways
- shrinks swollen membranes
-
WARNINGS
Do not use this product more than the recommended dosage, or if you are now taking a prescription Monoamine Oxidase Inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s Disease), or for 2 weeks after stopping the MAOI drug. If you are uncertain whether your prescription drug contains an MAOI, ask a health professional.
Ask a doctor before use if you have
- heart disease
- excessive phlegm (mucus)
- high blood pressure
- diabetes
- thyroid disease
- difficulty in urination due to enlargement of the prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- Directions
- Other Information
- INACTIVE INGREDIENT
- Questions?
- SPL UNCLASSIFIED SECTION
-
Giltuss TR
NDC 58552-317-02
GILTUSS ®
TOTAL RELEASE
EXPECTORANT, ANTITUSSIVE, AND NASAL DECONGESTANTSUGAR FREE AND PRESERVATIVE FREE
Each scored tablet contains:
Guaifenesin................................388 mg.
Dextromethorphan HBr................28 mg.
Phenylephrine HCl........................10 mg.
100 Tablets
MANUFACTURED FOR:
GIL PHARMACEUTICAL CORP.
PONCE, PUERTO RICO 00716

- Exactuss TR
-
INGREDIENTS AND APPEARANCE
GILTUSS TOTAL RELEASE
guaifenesin, dextromethorphan hbr, and phenylephrine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58552-317 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 388 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 28 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LIGHT MINERAL OIL (UNII: N6K5787QVP) POVIDONE K30 (UNII: U725QWY32X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white Score 2 pieces Shape OVAL Size 15mm Flavor Imprint Code GIL;GIL;303;303 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58552-317-02 12 in 1 CARTON 07/15/2011 1 2 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:58552-317-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2011 11/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 10/19/2009 EXACTUSS TOTAL RELEASE
guaifenesin, dextromethorphan hbr, and phenylephrine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58552-327 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 388 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 28 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LIGHT MINERAL OIL (UNII: N6K5787QVP) POVIDONE K30 (UNII: U725QWY32X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white Score 2 pieces Shape OVAL Size 15mm Flavor Imprint Code GIL;GIL;303;303 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58552-327-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 10/19/2009 Labeler - Gil Pharmaceutical Corp (176826592)




