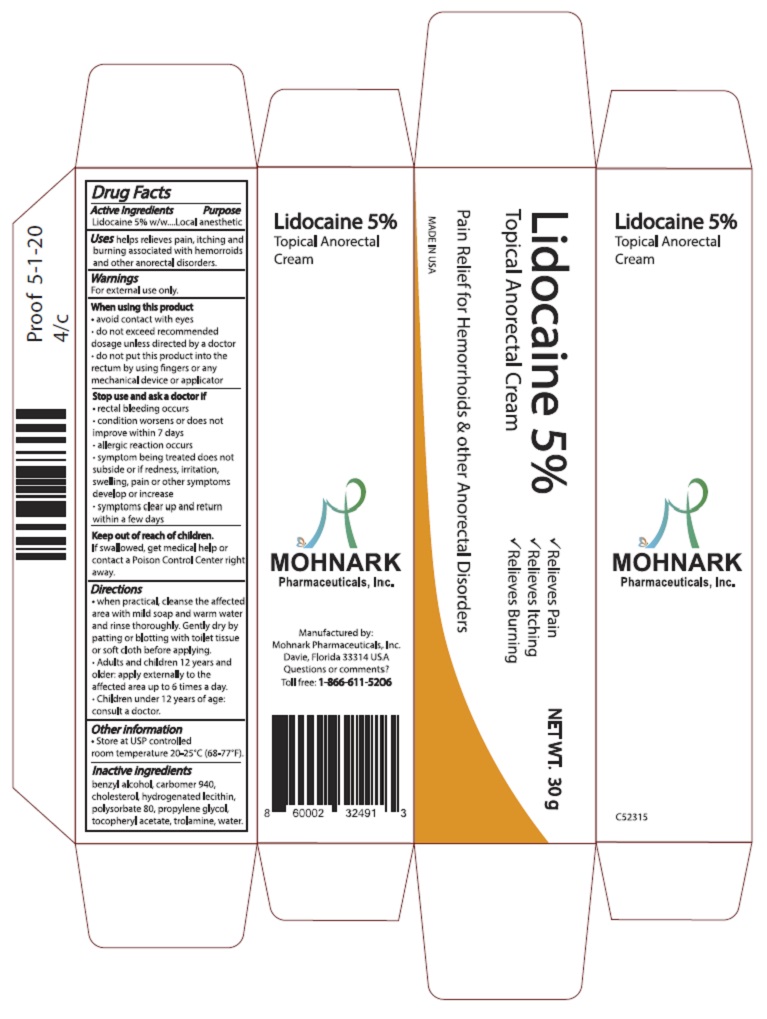
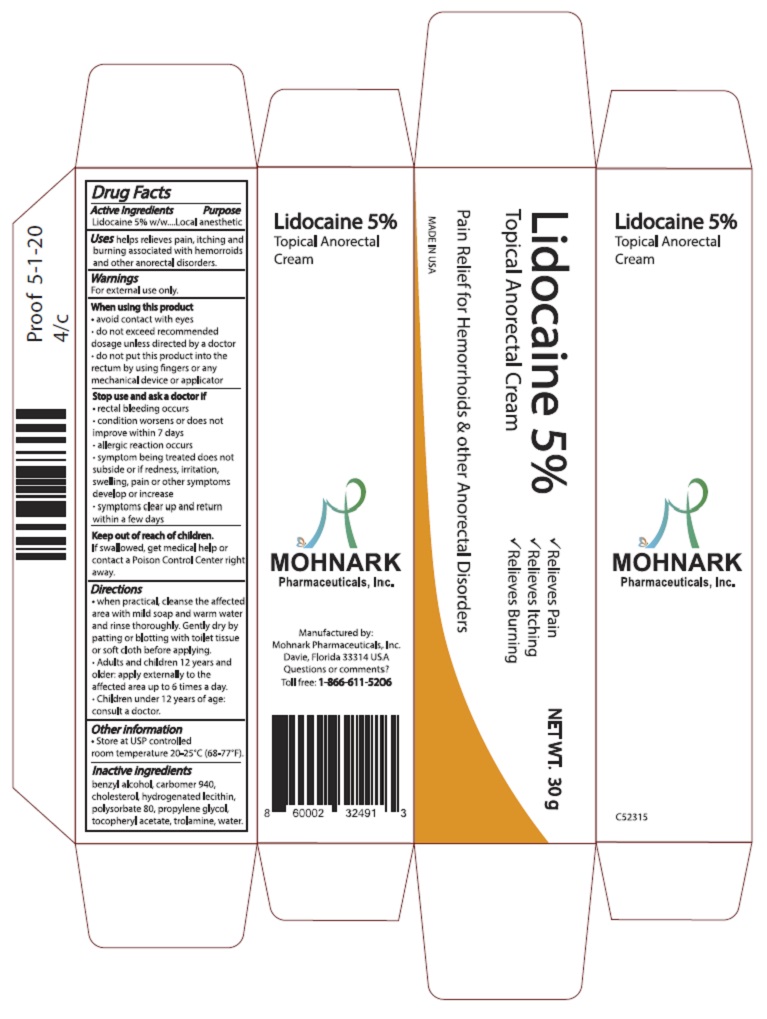
Label: LIDOCAINE 5% TOPICAL ANORECTAL CREAM cream
- NDC Code(s): 73715-002-01
- Packager: Mohnark Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Warnings
- When using this product
- Stop use and ask doctor if
- Keep out of reach of children
-
Directions
- when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or soft cloth before applying.
- Adults and children 12 years and older: apply externally to the affected area up to 6 times a day.
- Children under 12 years of age: consult a doctor.
- Other information
- Inactive ingredients
- Dosage
- Usage
- Package Label
-
INGREDIENTS AND APPEARANCE
LIDOCAINE 5% TOPICAL ANORECTAL CREAM
lidocaine 5% topical anorectal cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73715-002 Route of Administration RECTAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 5.25 g in 100 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) 2 g in 100 g WATER (UNII: 059QF0KO0R) 80 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73715-002-01 30 g in 1 TUBE; Type 0: Not a Combination Product 06/15/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 06/15/2020 Labeler - Mohnark Pharmaceuticals Inc. (117013830) Establishment Name Address ID/FEI Business Operations Mohnark Pharmaceuticals Inc. 117013830 manufacture(73715-002)