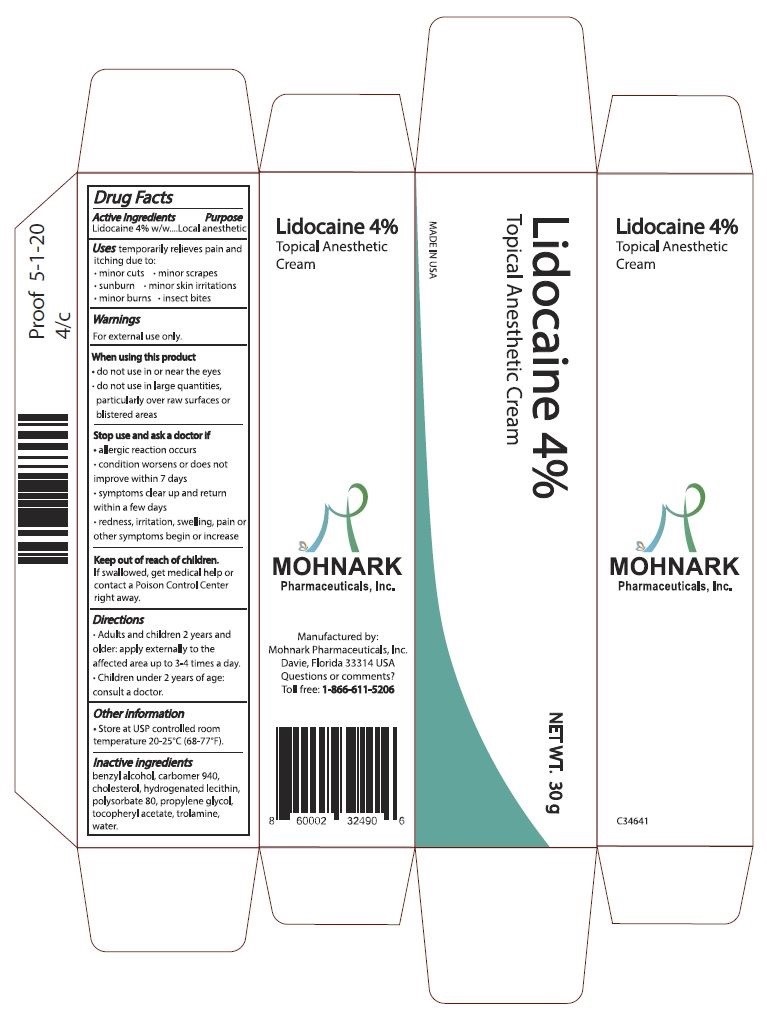
Label: LIDOCAINE 4% TOPICAL ANESTHETIC CREAM- lidocaine 4% cream cream
- NDC Code(s): 73715-001-01
- Packager: Mohnark Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Dosage
- Usage
- Package Label
-
INGREDIENTS AND APPEARANCE
LIDOCAINE 4% TOPICAL ANESTHETIC CREAM
lidocaine 4% cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73715-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4.27 g in 100 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) 2 g in 100 g WATER (UNII: 059QF0KO0R) 80.48 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73715-001-01 30 g in 1 TUBE; Type 0: Not a Combination Product 06/15/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/15/2020 Labeler - Mohnark Pharmaceuticals Inc. (117013830) Establishment Name Address ID/FEI Business Operations Mohnark Pharmaceuticals Inc. 117013830 manufacture(73715-001)