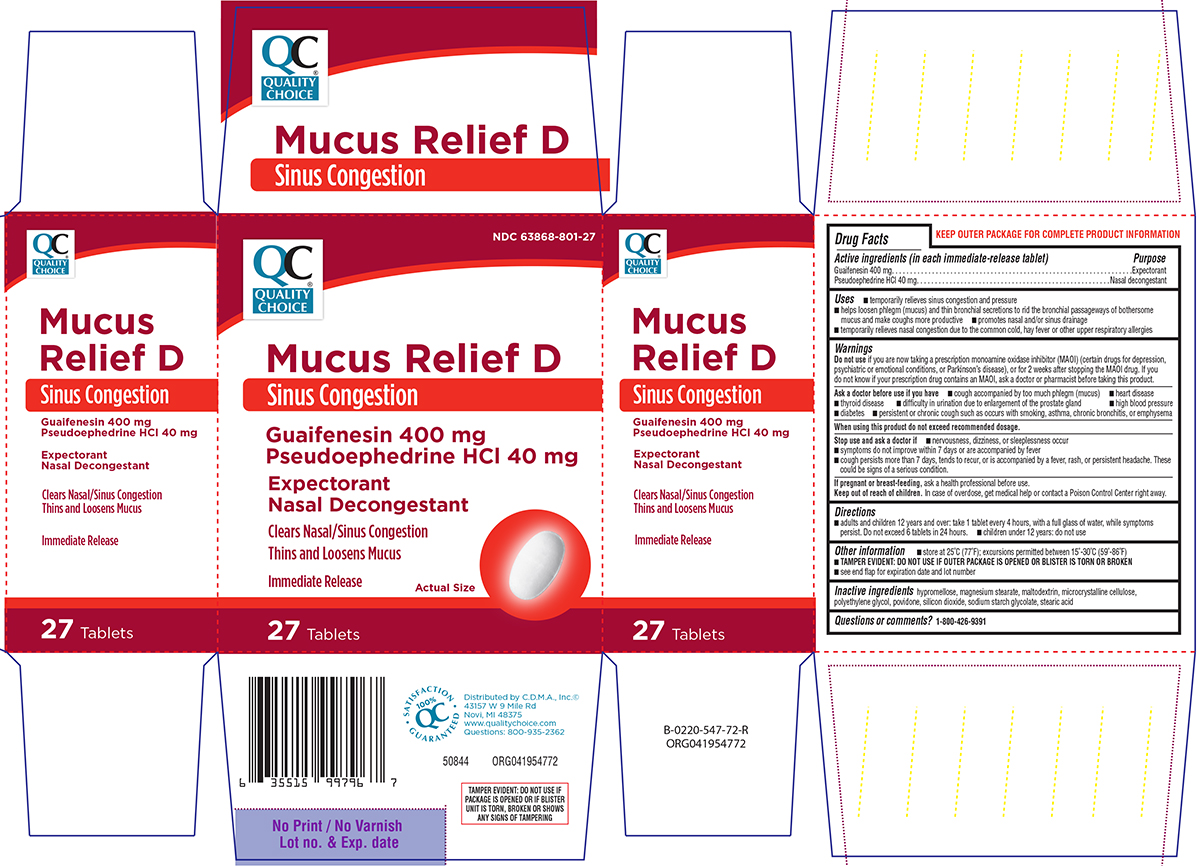
Label: MUCUS RELIEF D SINUS CONGESTION- guaifenesin, pseudoephedrine hcl tablet, film coated
- NDC Code(s): 63868-801-27
- Packager: CHAIN DRUG MARKETING ASSOCIATION INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each immediate-release tablet)
- Purpose
-
Uses
- temporarily relieves sinus congestion and pressure
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- promotes nasal and/or sinus drainage
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough accompanied by too much phlegm (mucus)
- heart disease
- thyroid disease
- difficulty in urination due to enlargement of the prostate gland
- high blood pressure
- diabetes
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
QC®
QUALITY
CHOICENDC 63868-801-27
Mucus Relief D
Sinus Congestion
Guaifenesin 400 mg
Pseudoephedrine HCl 40 mgExpectorant
Nasal DecongestantClears Nasal/Sinus Congestion
Thins and Loosens Mucus
Immediate Release
Actual Size
27 Tablets
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING50844 ORG041954772
SATISFACTION
100 %
QC
GUARANTEEDDistributed by C.D.M.A., Inc.©
43157 W 9 Mile Rd
Novi, MI 48375
www.qualitychoice.com
Questions: 800-935-2362
Quality Choice 44-547
-
INGREDIENTS AND APPEARANCE
MUCUS RELIEF D SINUS CONGESTION
guaifenesin, pseudoephedrine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-801 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 40 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score 2 pieces Shape OVAL Size 16mm Flavor Imprint Code 44;547 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-801-27 3 in 1 CARTON 04/23/2020 1 9 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/23/2020 Labeler - CHAIN DRUG MARKETING ASSOCIATION INC (011920774) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(63868-801) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 pack(63868-801) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(63868-801) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 pack(63868-801) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(63868-801)