Label: GREEN GUARD FIRST AID EYE WASH- water solution
-
NDC Code(s):
47682-410-08,
47682-410-11,
47682-410-18,
47682-410-26, view more47682-410-28, 47682-410-70, 47682-412-11, 47682-412-26
- Packager: Unifirst First Aid Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
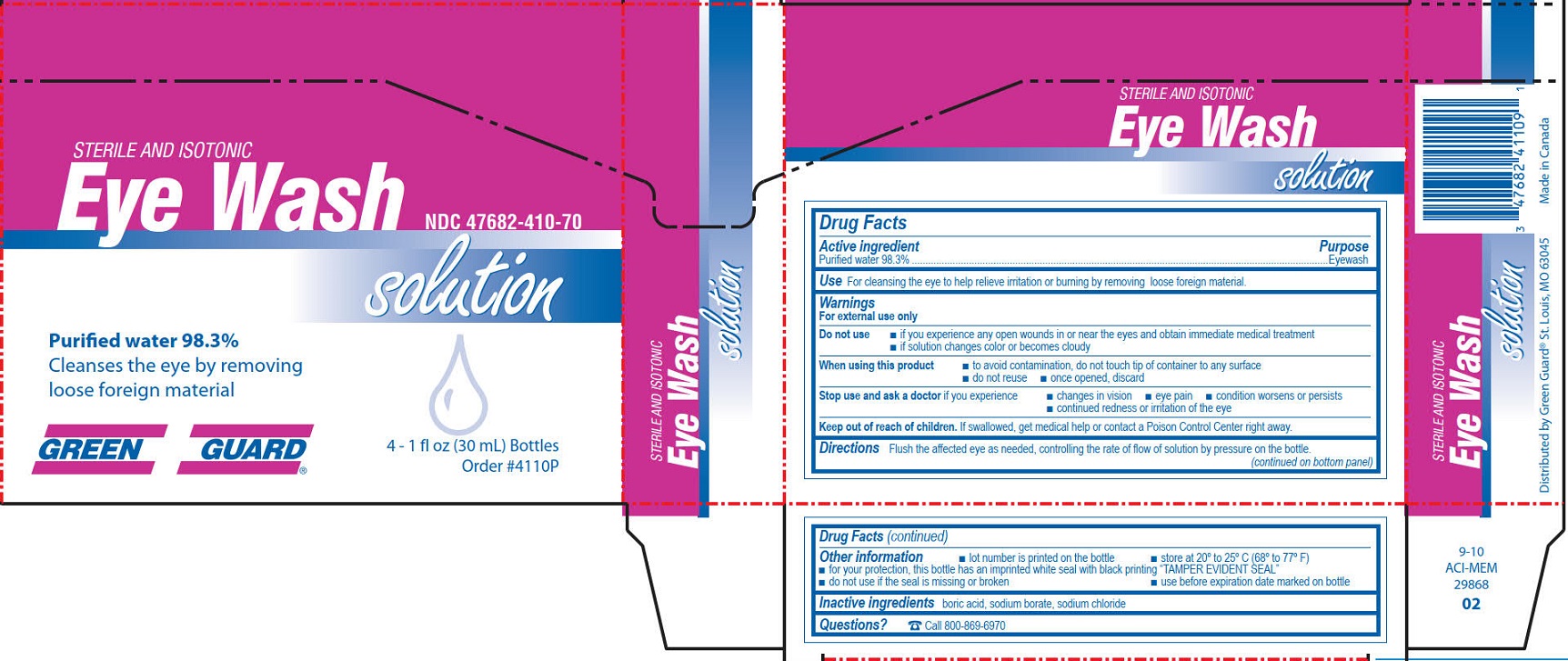
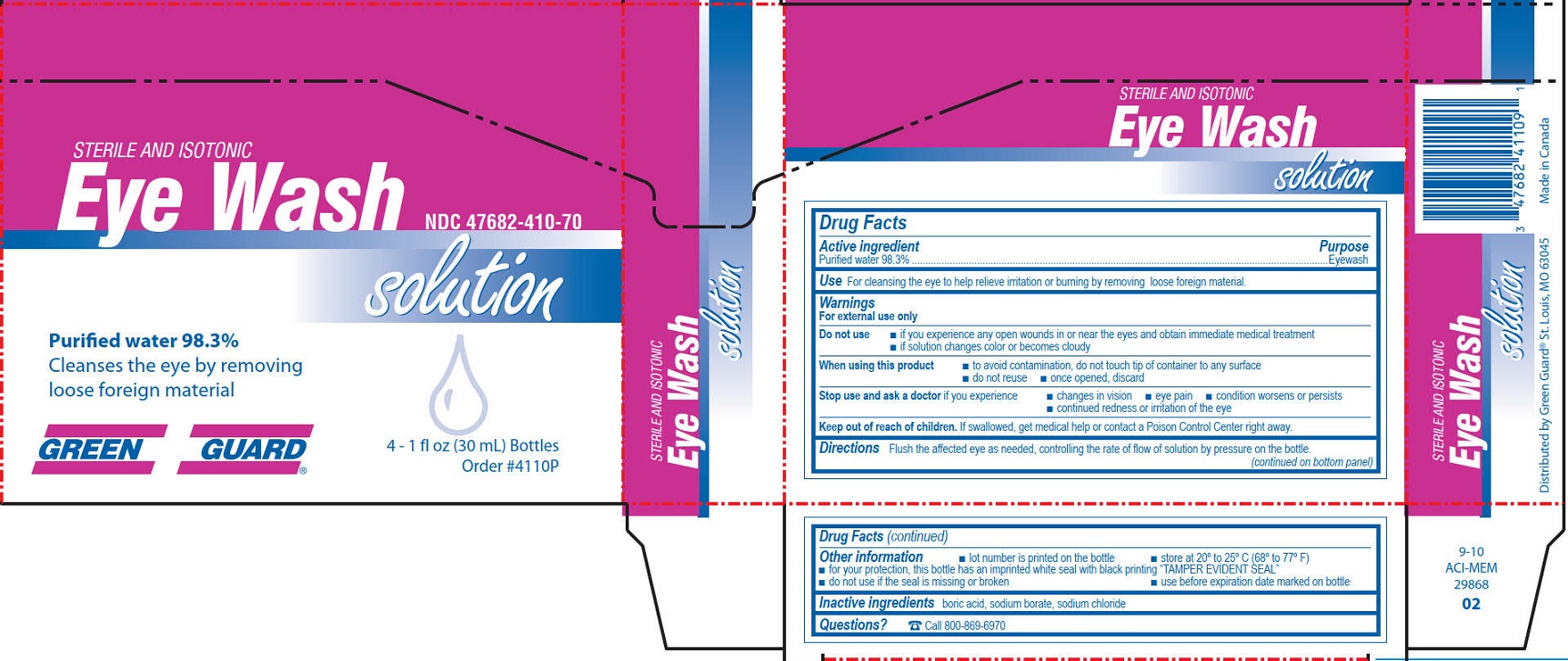
- Green Guard Eye Wash Label
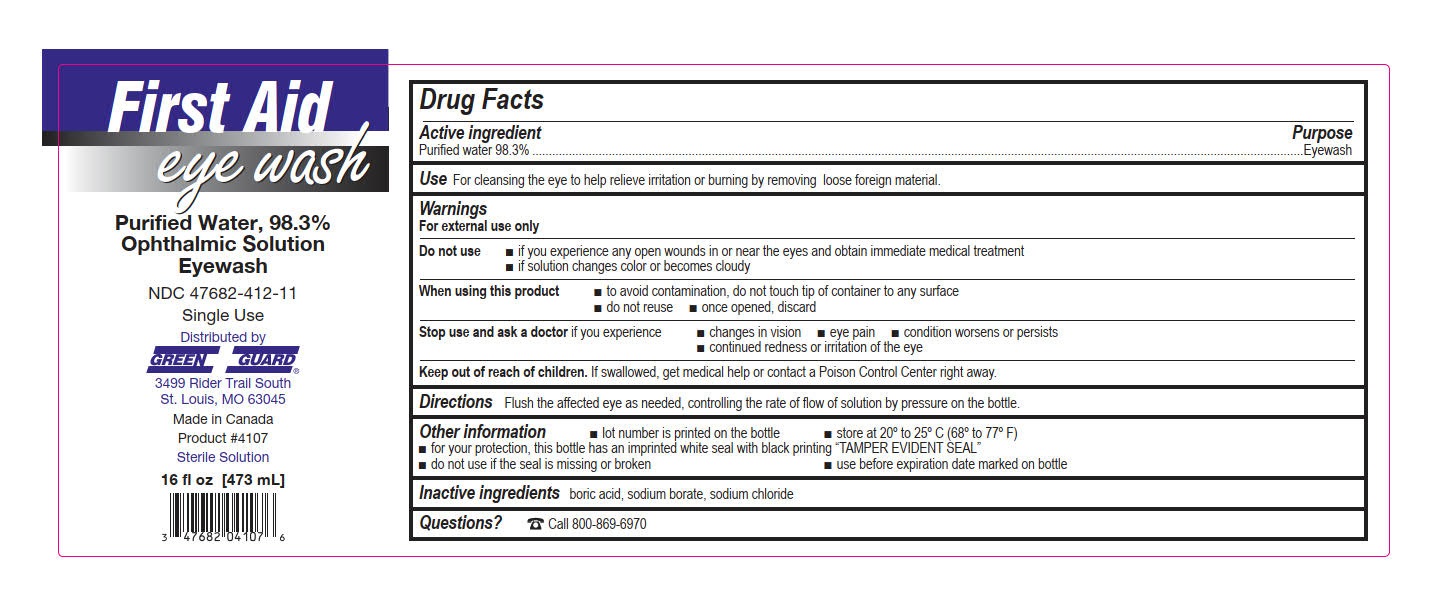
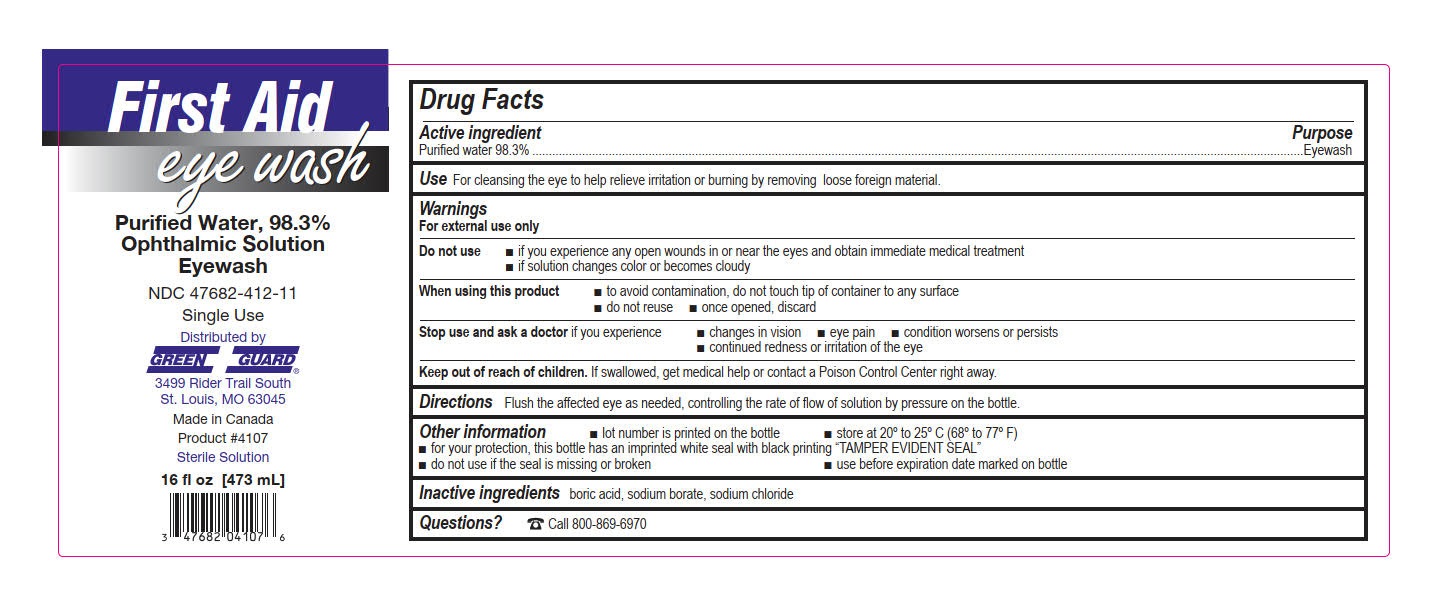
- Green Guard Eye Wash Label
-
INGREDIENTS AND APPEARANCE
GREEN GUARD FIRST AID EYE WASH
water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47682-410 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 0.983 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-410-28 30 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 12/02/2011 2 NDC:47682-410-18 118 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 12/02/2011 3 NDC:47682-410-08 236 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 12/02/2011 4 NDC:47682-410-11 473 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 12/02/2011 09/30/2015 5 NDC:47682-410-26 946 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 12/02/2011 03/30/2016 6 NDC:47682-410-70 4 in 1 BOX 12/02/2011 6 118 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 12/02/2011 GREEN GUARD FIRST AID EYE WASH
water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47682-412 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 0.983 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-412-11 473 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 10/01/2015 2 NDC:47682-412-26 946 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 04/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 10/01/2015 Labeler - Unifirst First Aid Corporation (832947092)