Label: DIAL COMPLETE LIMITED EDITION FHW MIDNIGHT TOAST solution
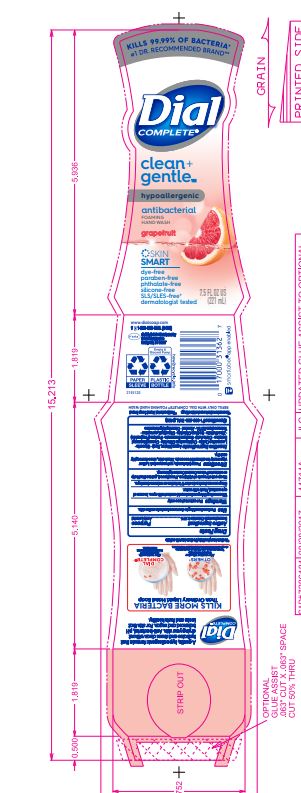
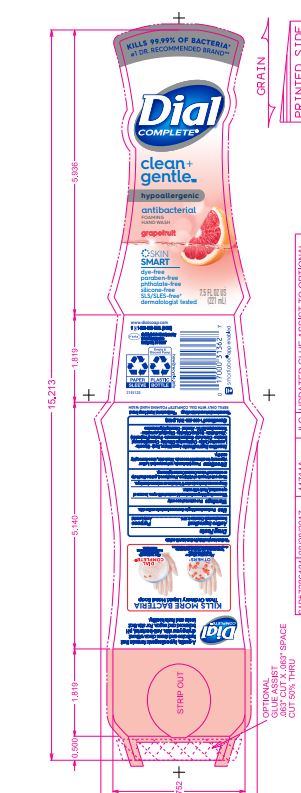
DIAL COMPLETE CG FHW GRAPEFRUIT solution
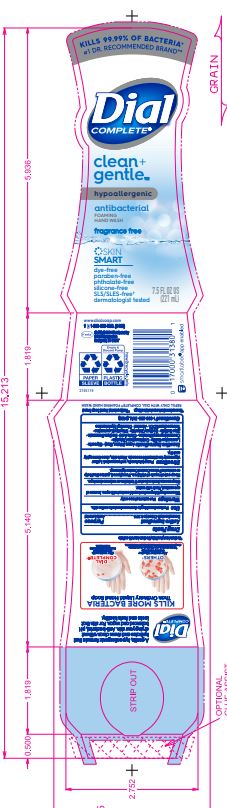
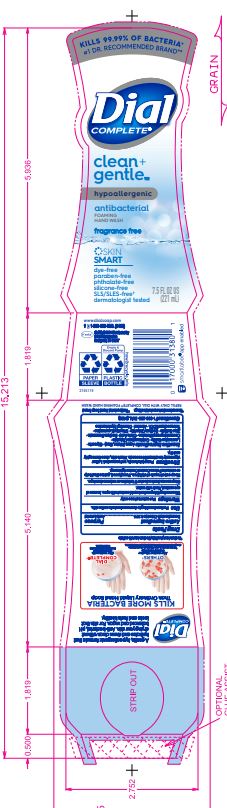
DIAL COMPLETE CG FHW FRAGRANCE FREE solution
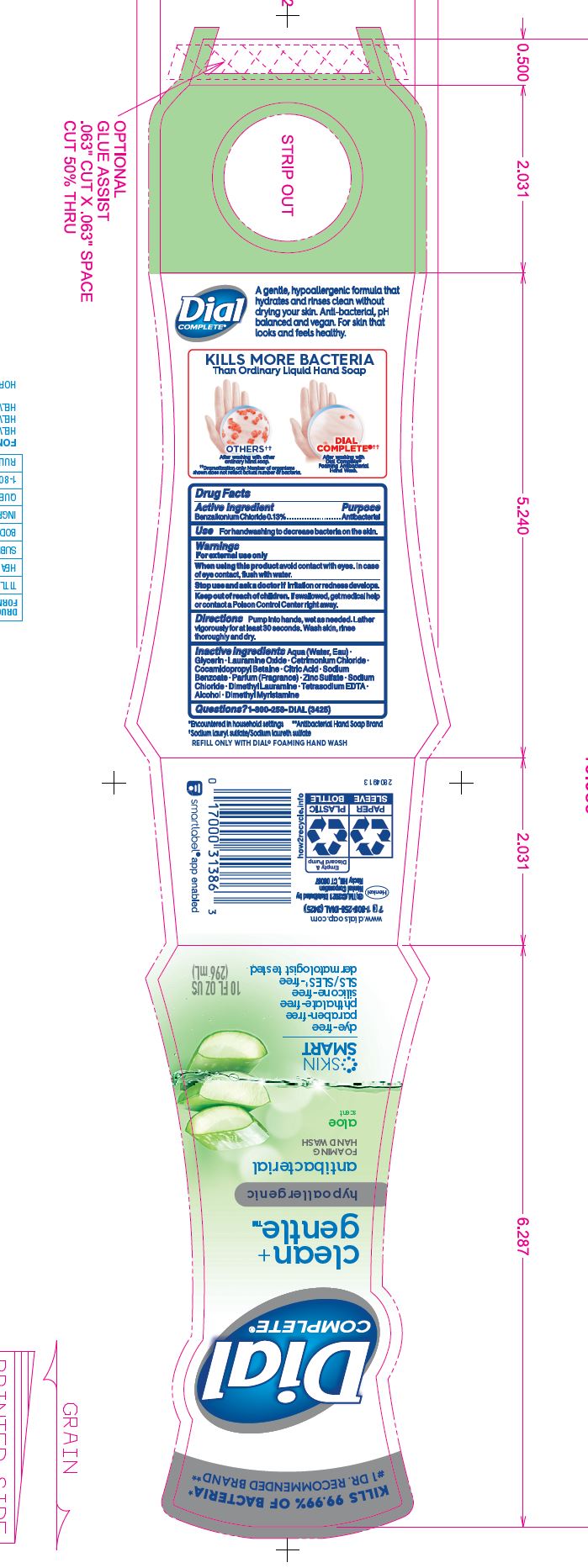
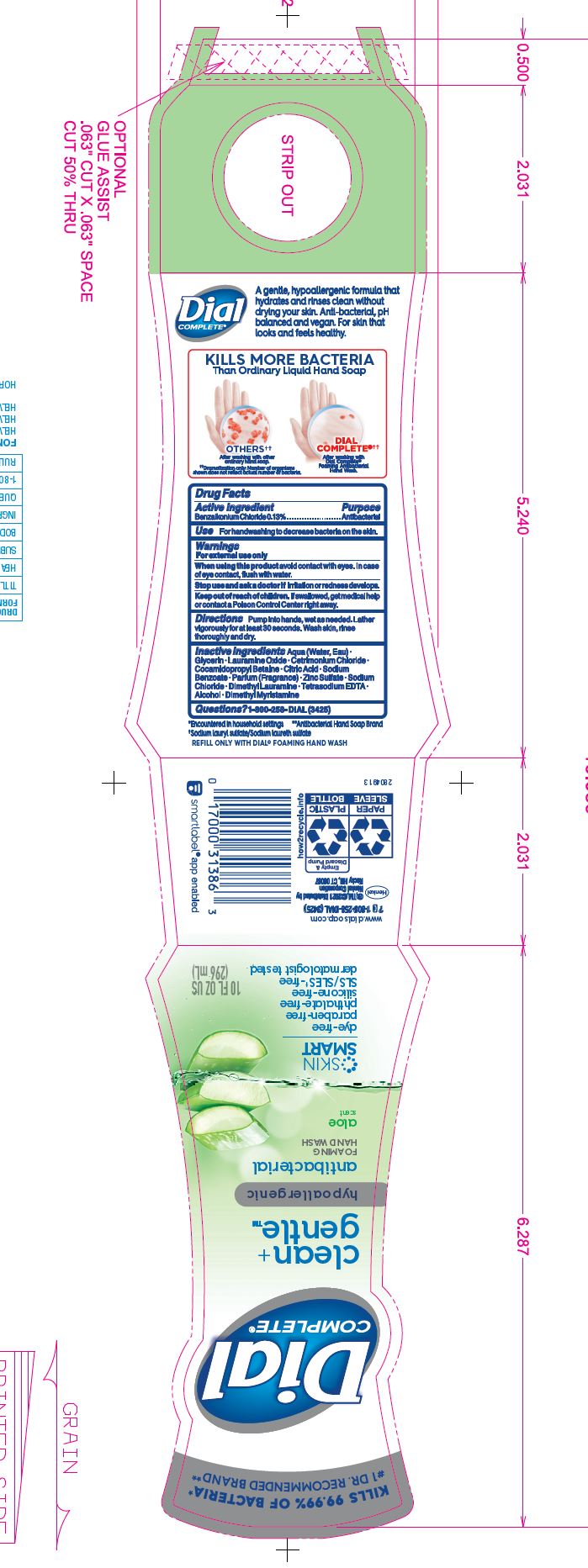
DIAL COMPLETE CG FHW ALOE solution
DIAL COMPLETE FHW COCONUT WATER solution
DIAL COMPLETE FHW FRESH LAVENDAR solution
DIAL COMPLETE 2IN1 FHW MINT AND SHEA solution
DIAL COMPLETE 2IN1 FHW PEARL ESSENCE solution
DIAL COMPLETE FRESH PEAR FHW solution
DIAL COMPLETE SPRING WATER FHW solution
DIAL COMPLETE FHW SOOTHING WHITE TEA solution
DIAL COMPLETE FHW POWER BERRIES solution
DIAL COMPLETE 2IN1 FHW MANUKA HONEY solution
DIAL COMPLETE 2IN1 FHW ROSE OIL solution
DIAL COMPLETE FHW CITRUS SUNBURST solution
DIAL COMPLETE FHW SILK AND SEABERRY solution
DIAL COMPLETE LIMITED EDITION FHW FIRESIDE CRACKLE solution
DIAL COMPLETE LIMITED EDITION FHW ICE CRYSTALS solution
-
NDC Code(s):
54340-149-01,
54340-149-02,
54340-149-03,
54340-149-04, view more54340-149-05, 54340-150-01, 54340-150-02, 54340-150-03, 54340-150-04, 54340-150-05, 54340-151-01, 54340-151-02, 54340-152-01, 54340-152-02, 54340-152-03, 54340-152-04, 54340-153-01, 54340-153-02, 54340-153-03, 54340-154-01, 54340-154-02, 54340-155-01, 54340-155-02, 54340-156-02, 54340-156-03, 54340-157-01, 54340-158-01, 54340-159-01, 54340-160-01, 54340-161-04, 54340-162-05, 54340-163-06, 54340-163-07, 54340-168-02, 54340-169-03, 54340-170-04
- Packager: Henkel Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Purpose
- Uses
- WARNINGS
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
-
INACTIVE INGREDIENT
Spring Water
Inactive Ingredients: Aqua (Water, Eau) · Glycerin · Lauramine Oxide · Cetrimonium Chloride · Cocamidopropyl Betaine ·
Citric Acid · Sodium Benzoate · Hydroxypropyl Methylcellulose · Parfum (Fragrance) · Zinc Sulfate · Sodium Chloride·
Dimethyl Lauramine · Tetrasodium EDTA · Alcohol· Dimethyl Myristamine · Cl 42090 (Blue 1) · Cl 17200 (Red 33)Citrus
Inactive Ingredients: Aqua (Water, Eau) · Glycerin · Lauramine Oxide · Cetrimonium Chloride · Cocamidopropyl Betaine ·
Citric Acid · Parfum (Fragrance) · Sodium Benzoate · Hydroxypropyl Methylcellulose · Zinc Sulfate· Sodium Chloride ·
Dimethyl Lauramine · Tetrasodium EDTA · Alcohol· Dimethyl Myristamine · Cl 14700 (Red 4) · Cl 15985 (Yellow 6)
Coconut Water
Inactive Ingredients: Aqua (Water, Eau) · Glycerin · Lauramine Oxide · Cetrimonium Chloride · Cocamidopropyl Betaine ·
Citric Acid · Sodium Benzoate · Parfum (Fragrance) · Hydroxypropyl Methylcellulose · Zinc Sulfate · Sodium Chloride ·
Dimethyl Lauramine · Tetrasodium EDTA · Alcohol· Dimethyl Myristamine · Cl 42090 (Blue 1) · Cl 17200 (Red 33)Lavendar Jasmine
Inactive Ingredients: Aqua (Water, Eau) · Glycerin · Lauramine Oxide · Cetrimonium Chloride · Cocamidopropyl Betalne ·
Citric Acid · Parfum (Fragrance) · Sodium Benzoate · Hydroxypropyl Methylcellulose · Zinc Sulfate· Sodium Chloride ·
Dimethyl Lauramine · Tetrasodium EDTA · Alcohol · Dimethyl Myristamlne · Cl 60730 (Ext. Violet 2)Fresh Pear
Inactive Ingredients: Aqua (Water, Eau) · Glycerin · Lauramine Oxide · Cetrimonium Chloride · Cocamidopropyl Betaine •
Citric Acid • Sodium Benzoate · Hydroxypropyl Methylcellulose · Parfum (Fragrance)· Zinc Sulfate· Sodium Chloride•
Dimethyl Lauramine · Tetrasodium EDTA · Alcohol · Dimethyl Myristamine , Cl 47005 (Yellow 1 0) · Cl 42053 (Green 3)Power Berries
nactive Ingredients: Aqua (Waler, Eau) · Glycerin · Lauramine Oxide · Cetrimonium Chloride · Cocamldopropyl Betaine ·
Citric Acid · Sodium Benzoate · Parfum (Fragrance) · Hydroxypropyl Methylcellulose · Zinc Sulfate · Sodium Chloride ·
Dimethyl Lauramine · Tetrasodium EDTA · Alcohol · Dimethyl Myristamine · Tocopheryl Acetate ·
Vaccinium Macrocarpon (Cranberry) Fruit Juice · Punica Granatum Fruit Juice · Rubus ldaeus (Raspberry) Juice·
Cl 17200 (Red 33) · Cl 15510 (Orange 4)Silk and Seaberry
Inactive Ingredients: Aqua (Waler, Eau) · Glycerin · Lauramine Oxide · Cetrimonium Chloride · Cocamidopropyl Betaine ·
Citric Acid · Sodium Benzoate · Parfum (Fragrance) · Hydroxypropyl Methylcellulose · Zinc Sulfate· Sodium Chloride·
Dimethyl Lauramine · Tetrasodium EDTA, Alcohol· Dimethyl Myristamine · Cl 15985 (Yellow 6) · Cl 14700 (Red 4)
White Tea
Inactive Ingredients: Aqua (Water, Eau) · Glycerin · Lauramine Oxide · Cetrimonium Chloride · Cocamidopropyl Betaine ·
Citric Acid · Sodium Benzoate · Hydroxypropyl Methylcellulose · Parfum (Fragrance)· Zinc Sulfate· Sodium Chloride·
Dimethyl Lauramine · Tetrasodium EDTA · Alcohol· Dimethyl Myristamine · Cl 42090 (Blue 1) · Cl 17200 (Red 33)2in1 Manuka Honey
Inactive Ingredients: Aqua (Water, Eau) · Lauramidopropylamine Oxide · Glycerin · Cetrimonium Chloride · Lauramine Oxide ·
Citric Acid · Cocamidopropyl Betaine · Parfum (Fragrance) · Trideceth-9 · Sodium Benzoate · Myristamidopropylamine Oxide ·
Hydroxypropyl Methylcellulose · PEG-5 lsononanoate · Zinc Sulfate· Sodium Chloride· Dimethyl Lauramine · Tetrasodium EDTA
·Alcohol· Dimethyl Myristamine · Mel (Honey)· Cl 19140 (Yellow 5) · Cl 14700 (Red 4)2in1 Mint Shea
Inactive Ingredients: Aqua (Water, Eau) · Lauramidopropylamine Oxide · Cetrimonium Chloride · Glycerin · Lauramine Oxide ·
Citric Acid · Cocamidopropyl Betaine · Trideceth-9 · Sodium Benzoate · Parfum (Fragrance) · Myristamidopropylamine Oxide ·
Hydroxypropyl Methylcellulose · PEG-5 lsononanoate · Zinc Sulfate· Sodium Chloride· Dimethyl Lauramine · Tetrasodium EDTA
·Alcohol· Dimethyl Myristamine · Cl 42090 (Blue 1) · Cl 47005 (Yellow 10)2in1 Pearl Essence
Inactive Ingredients: Aqua (Water, Eau) · Lauramidopropylamine Oxide · Glycerin · Cetrimonium Chloride · Lauramine Oxide ·
Citric Acid · Cocamidopropyl Betaine · Trideceth-9 · Sodium Benzoate · Parfum (Fragrance) · Myristamidopropylamine Oxide ·
Hydroxypropyl Methylcellulose· PEG-5 lsononanoate · Zinc Sulfate· Sodium Chloride· Dimethyl Lauramine · Tetrasodium EDTA ·
Alcohol· Dimethyl Myristamine · Maris Sal (Sea Salt)· Hydrolyzed Pearl· Cl 42090 (Blue 1) · Cl 17200 (Red 33)
2in1 Rose Oil
Inactive Ingredients: Aqua (Water, Eau) · Lauramidopropylamine Oxide · Glycerin · Cetrimonium Chloride · Lauramine Oxide ·
Citric Acid · Cocamidopropyl Betaine · Trideceth-9 · Sodium Benzoate · Parfum (Fragrance) · Myristamidopropylamine Oxide ·
Hydroxypropyl Methylcellulose· PEG-5 lsononanoate · Zinc Sulfate· Sodium Chloride· Dimethyl Lauramine · Tetrasodium EDTA ·
Alcohol· Dimethyl Myristamine · Rosa Damascena Flower Oil· Cl 15985 (Yellow 6) · Cl 17200 (Red 33) · Cl 16035 (Red 40)Clean Gentle FHW Aloe
Inactive Ingredients: Aqua (Water, Eau)· Glycerin · Lauramine Oxide · Cetrimonium Chloride ·
Cocamidopropyl Betaine · Citric Acid, Sodium Benzoate · Parfum (Fragrance) · Zinc Sulfate· Sodium Chloride· Dimethyl Lauramine · Tetrasodium EDTA ·Alcohol· Dimethyl MyristamineClean Gentle Grapefruit
Inactive Ingredients: Aqua (Water, Eau)· Glycerin · Lauramine Oxide · Cetrimonium Chloride ·
Cocamidopropyl Betaine · Citric Acid, Sodium Benzoate · Parfum (Fragrance) · Zinc Sulfate· Sodium Chloride· Dimethyl Lauramine · Tetrasodium EDTA ·Alcohol· Dimethyl MyristamineClean Gentle FHW Fragrance Free
Inactive Ingredients: Aqua (Water, Eau)· Glycerin · Lauramine Oxide · Cetrimonium Chloride ·
Cocamidopropyl Betaine · Citric Acid, Sodium Benzoate · Zinc Sulfate· Sodium Chloride· Dimethyl Lauramine · Tetrasodium EDTA ·Alcohol· Dimethyl MyristamineDial Complete Limited Edition FHW Midnight Toast
Aqua (Water, Eau)· Glycerin · Lauramine Oxide · Cetrimonium Chloride ·
Cocamidopropyl Betaine · Citric Acid, Sodium Benzoate · Parfum (Fragrance)· Zinc Sulfate· Sodium Chloride· Dimethyl Lauramine · Tetrasodium EDTA ·Alcohol· Dimethyl MyristamineDial Complete Limited Edition FHW Ice Crystals
Aqua (Water, Eau)· Glycerin · Lauramine Oxide · Cetrimonium Chloride ·
Cocamidopropyl Betaine · Citric Acid, Sodium Benzoate · Parfum (Fragrance)· Zinc Sulfate· Sodium Chloride· Dimethyl Lauramine · Tetrasodium EDTA ·Alcohol· Dimethyl MyristamineDial Complete Limited Edition FHW Fireside Crackle
Aqua (Water, Eau)· Glycerin · Lauramine Oxide · Cetrimonium Chloride ·
Cocamidopropyl Betaine · Citric Acid, Sodium Benzoate · Parfum (Fragrance)· Zinc Sulfate· Sodium Chloride· Dimethyl Lauramine · Tetrasodium EDTA ·Alcohol· Dimethyl Myristamine - SPL UNCLASSIFIED SECTION
- Questions
- Legal entity
- Refill Instruction
- INDICATIONS & USAGE
- Topical
- Principle display
-
INGREDIENTS AND APPEARANCE
DIAL COMPLETE LIMITED EDITION FHW MIDNIGHT TOAST
dial complete limited edition fhw midnight toast solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-170 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.23 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-170-04 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE CG FHW GRAPEFRUIT
dial complete cg fhw grapefruit solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-162 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.23 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-162-05 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE CG FHW FRAGRANCE FREE
dial complete cg fhw fragrance free solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-163 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.23 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-163-06 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-163-07 325 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE CG FHW ALOE
dial complete cg fhw aloe solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-161 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.23 mL in 100 mL Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-161-04 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 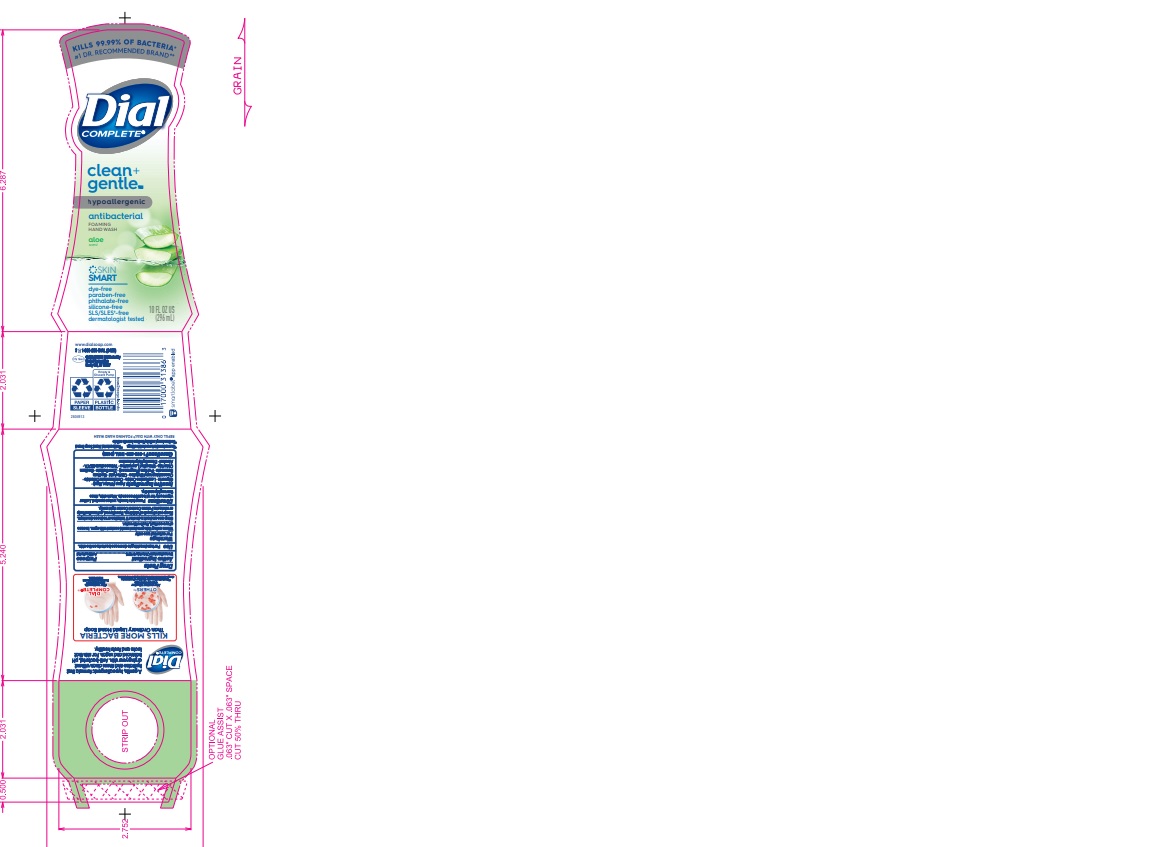
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE FHW COCONUT WATER
dial complete fhw coconut water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-153 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.23 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-153-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-153-02 296 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 3 NDC:54340-153-03 444 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE FHW FRESH LAVENDAR
dial complete fhw fresh lavendar solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-151 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.01 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-151-01 296 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-151-02 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE 2IN1 FHW MINT AND SHEA
dial complete 2in1 fhw mint and shea solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-160 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 91.24 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-160-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE 2IN1 FHW PEARL ESSENCE
dial complete 2in1 fhw pearl essence solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-159 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 91.15 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-159-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE FRESH PEAR FHW
dial complete fresh pear fhw solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-150 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.3 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-150-02 946 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-150-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 3 NDC:54340-150-03 1180 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 4 NDC:54340-150-04 296 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 5 NDC:54340-150-05 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE SPRING WATER FHW
dial complete spring water fhw solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-149 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.27 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-149-02 946 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-149-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 3 NDC:54340-149-03 1180 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 4 NDC:54340-149-04 296 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 5 NDC:54340-149-05 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE FHW SOOTHING WHITE TEA
dial complete fhw soothing white tea solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-152 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.32 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-152-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-152-02 296 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 3 NDC:54340-152-03 946 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 4 NDC:54340-152-04 444 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE FHW POWER BERRIES
dial complete fhw power berries solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.19 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-155-02 296 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-155-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE 2IN1 FHW MANUKA HONEY
dial complete 2in1 fhw manuka honey solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-157 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 91.05 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-157-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE 2IN1 FHW ROSE OIL
dial complete 2in1 fhw rose oil solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-158 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 91.22 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-158-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE FHW CITRUS SUNBURST
dial complete fhw citrus sunburst solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-156 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.03 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-156-02 296 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-156-03 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE FHW SILK AND SEABERRY
dial complete fhw silk and seaberry solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-154 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.23 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-154-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-154-02 296 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE LIMITED EDITION FHW FIRESIDE CRACKLE
dial complete limited edition fhw fireside crackle solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-168 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.23 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-168-02 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE LIMITED EDITION FHW ICE CRYSTALS
dial complete limited edition fhw ice crystals solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-169 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 94.23 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-169-03 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 Labeler - Henkel Corporation (080887708)