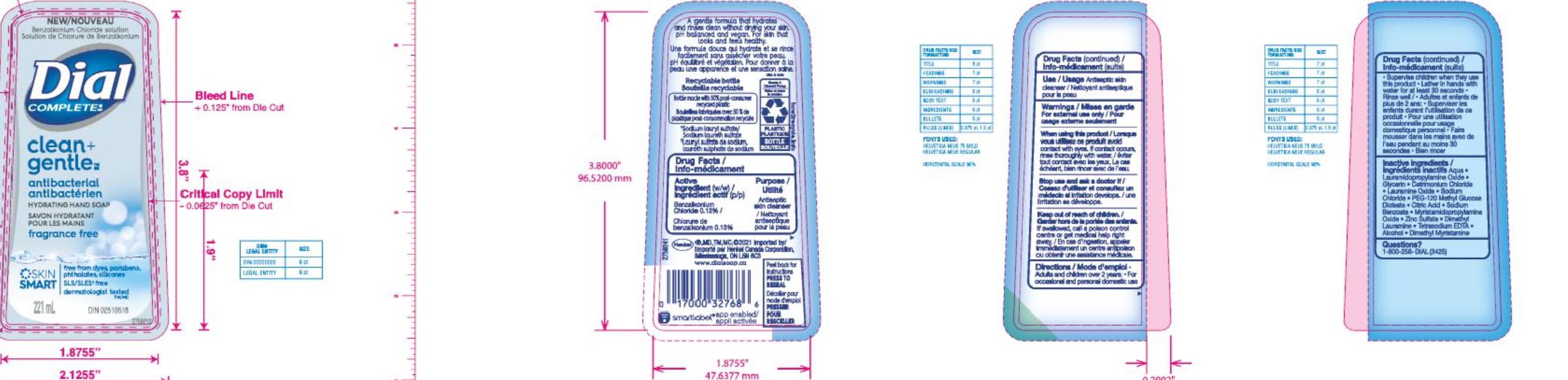
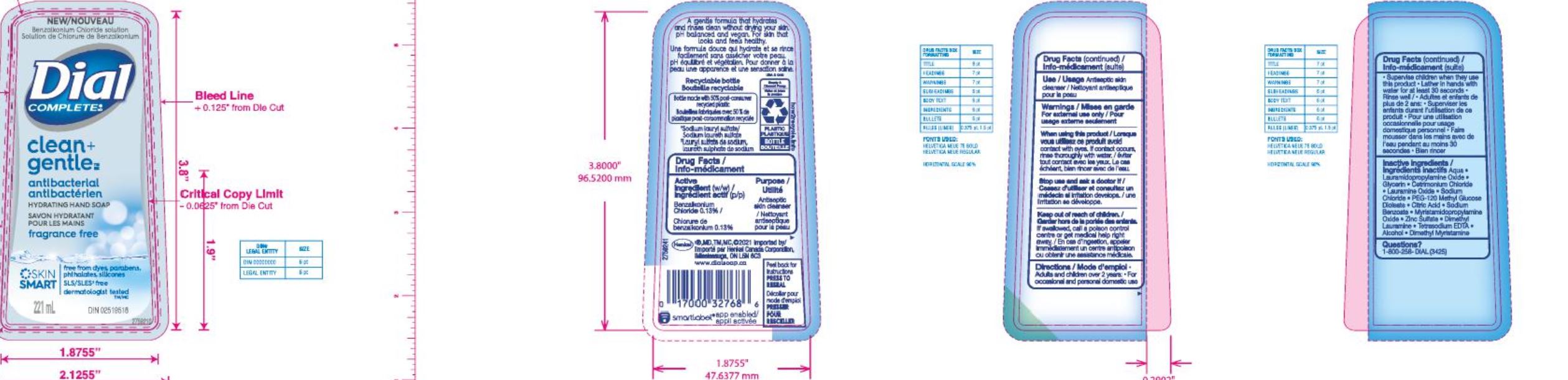
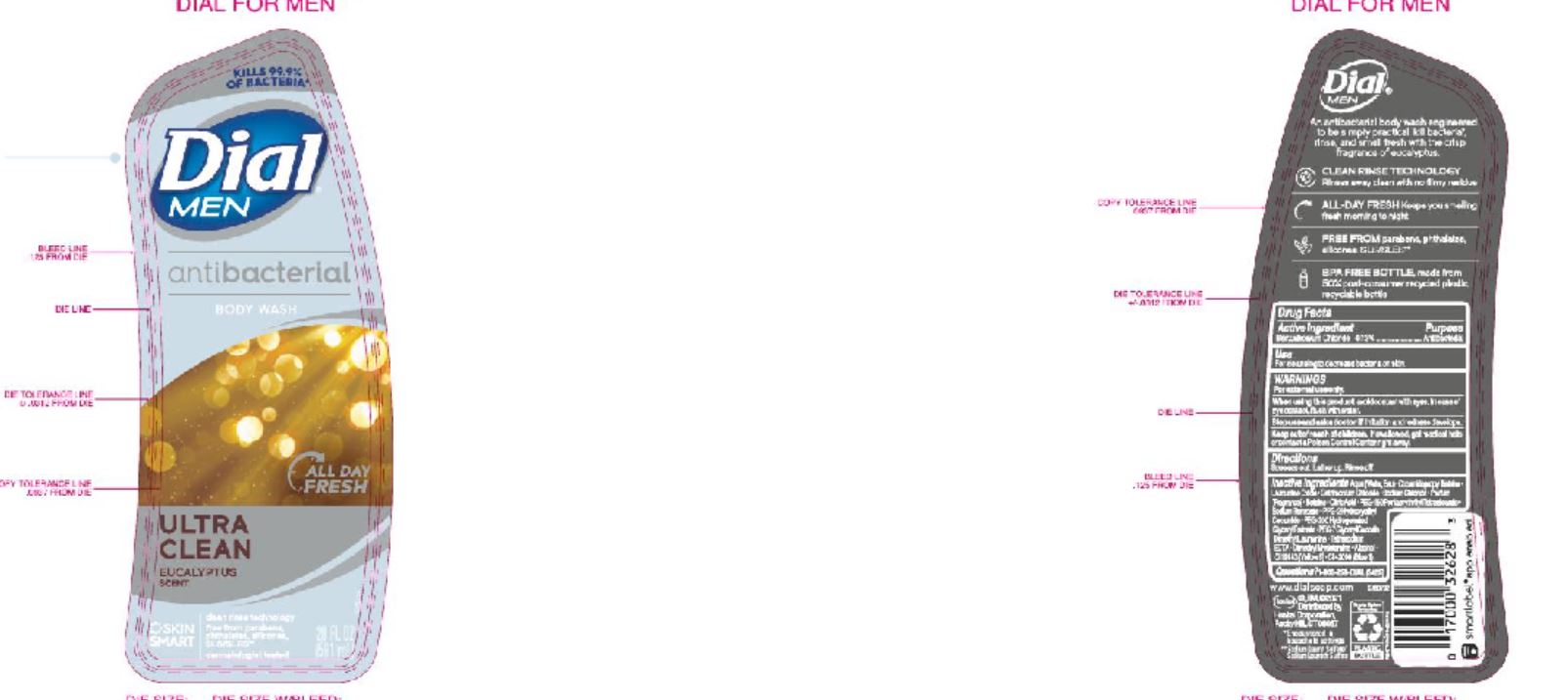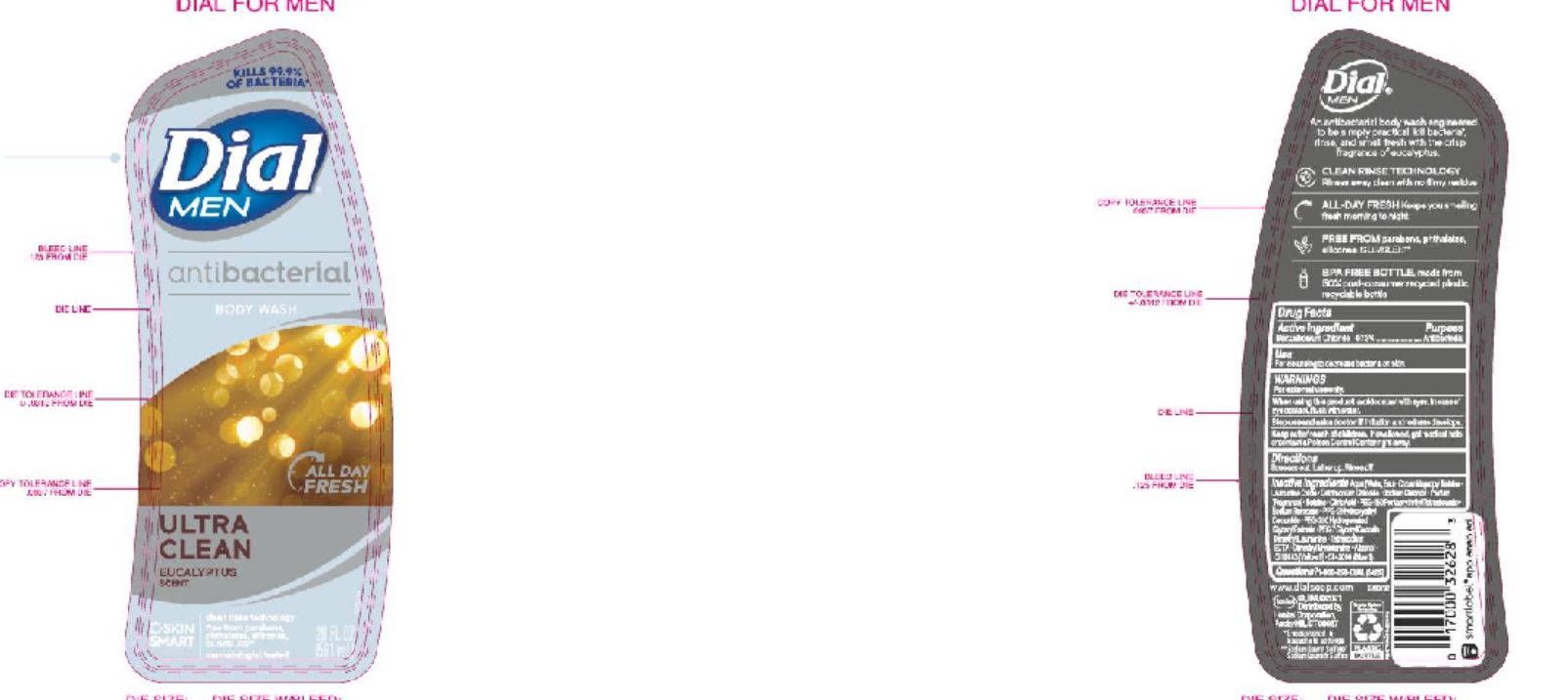Label: DIAL COMPLETE LHS CLEAN AND GENTLE- liquid hand soap clean and gentle solution
DIAL COMPLETE LHS LAVENDER AND JASMINE- liquid hand soap lavender and jasmine solution
DIAL COMPLETE ALOE- antibacterial hand wash aloe solution
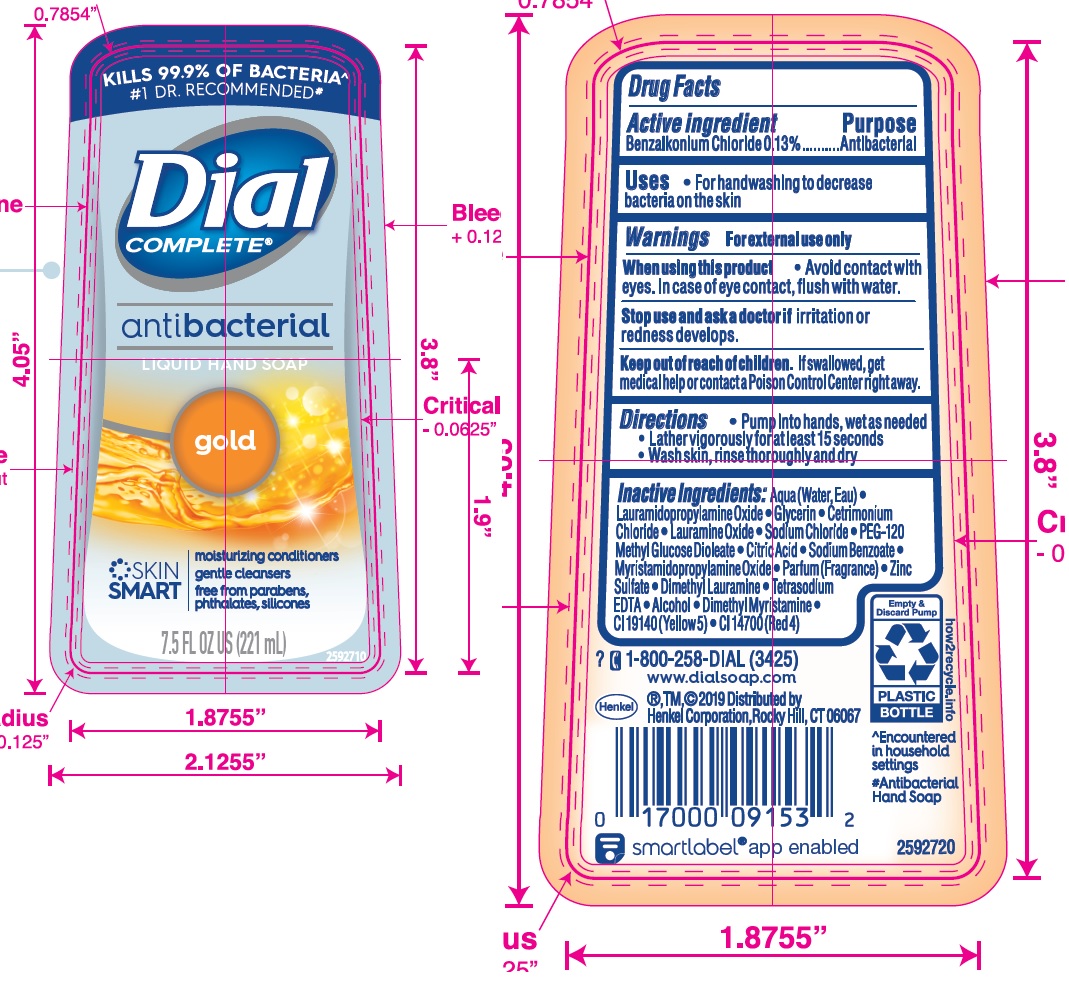
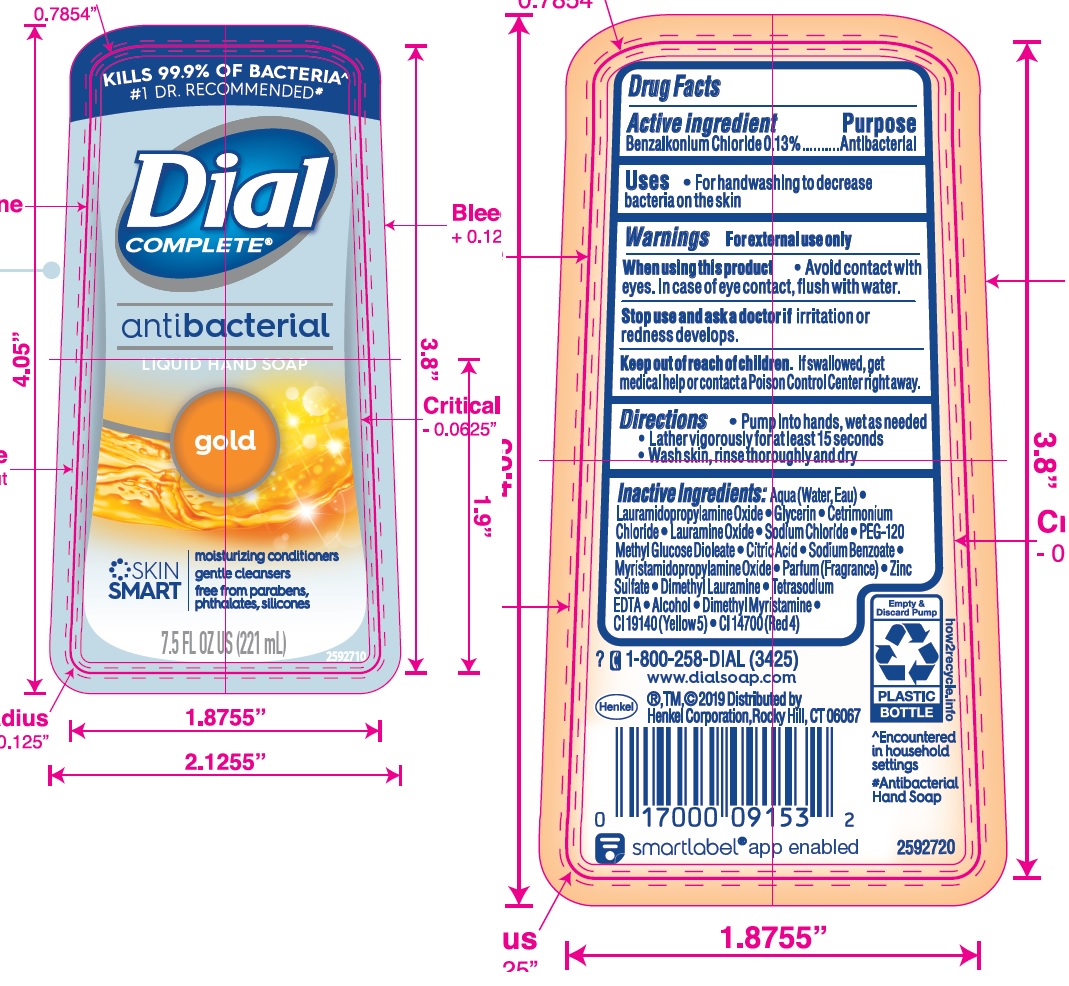
DIAL COMPLETE GOLD- antibacterial hand wash gold solution
DIAL COMPLETE SPRING WATER- antibacterial hand wash spring water solution
DIAL COMPLETE WHITE TEA- antibacterial hand wash white tea solution
DIAL COMPLETE LHS LEMON AND SAGE- liquid hand soap lemon and sage solution
DIAL COMPLETE LHS SWEET WATERMELON- liquid hand soap sweet watermelon solution
DIAL COMPLETE LHS POMEGRANATE AND TANGERINE- liquid hand soap pomegranate and tangerine solution
DIAL COMPLETE LHS CLEAN AND GENTLE- liquid hand soap waterlilly solution
DIAL COMPLETE BW MEN solution
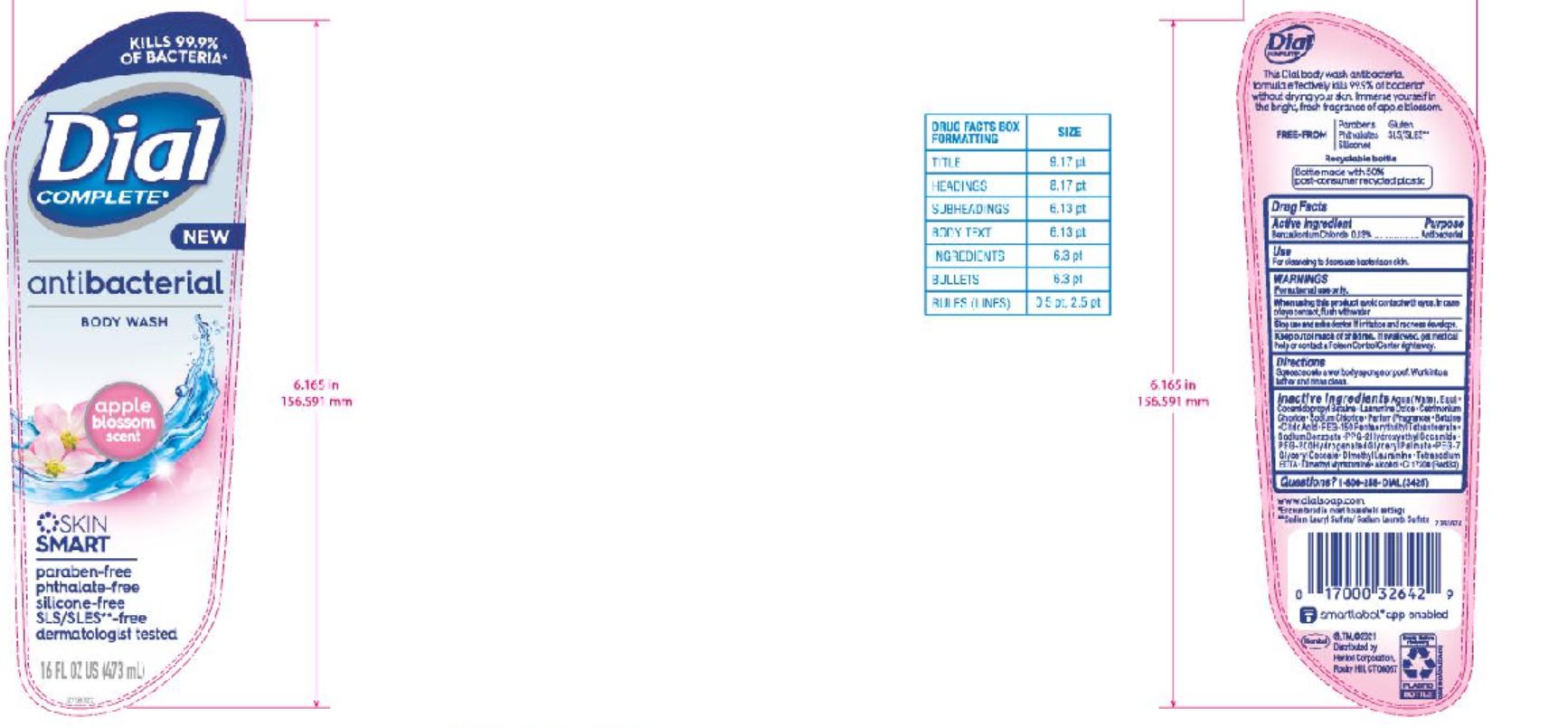
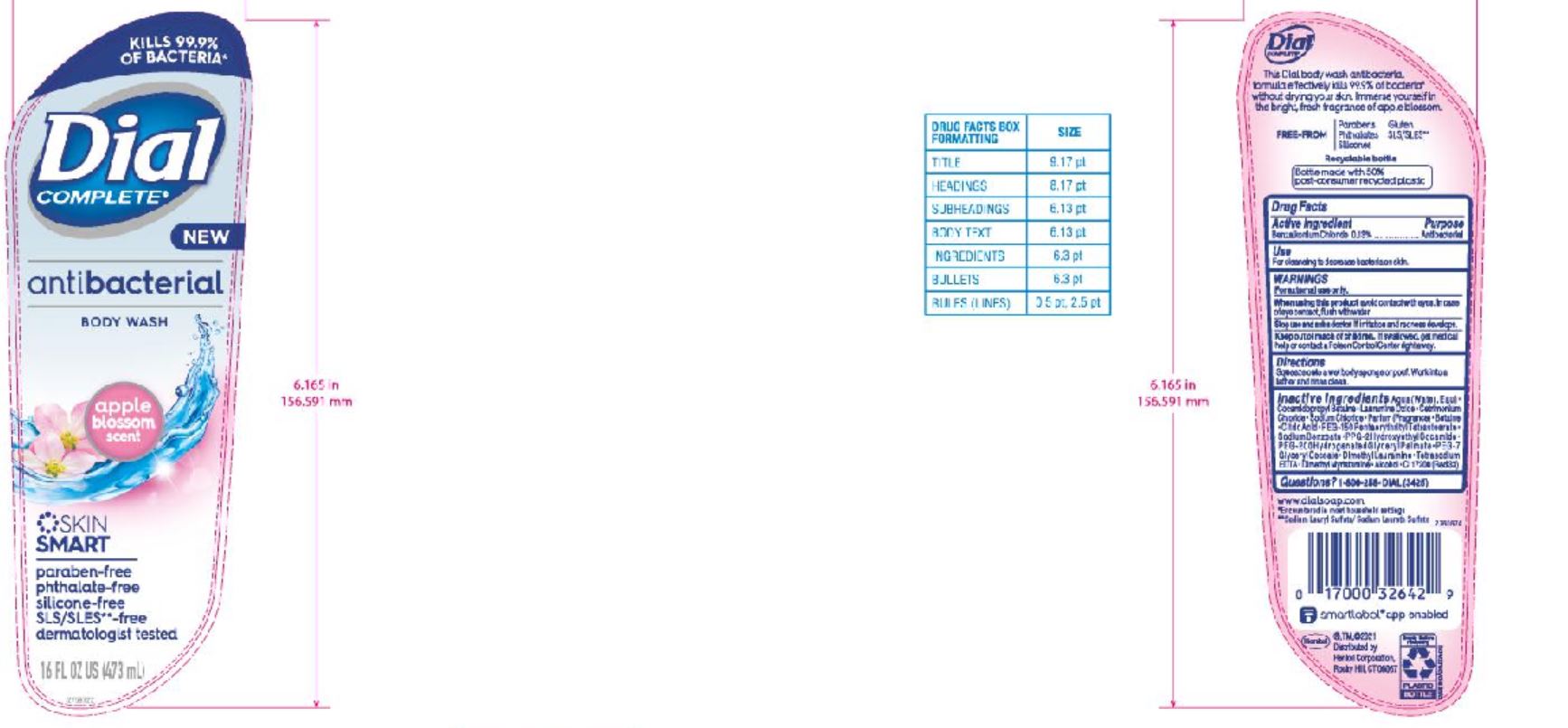
DIAL COMPLETE BW APPLE BLOSSOM solution
DIAL ADVANCED CLEAN ANTIBACTERIAL BODY WASH APPLE BLOSSOM- benzalkonium chloride solution
-
NDC Code(s):
54340-141-01,
54340-141-02,
54340-142-02,
54340-142-03, view more54340-142-04, 54340-142-05, 54340-143-01, 54340-143-02, 54340-143-03, 54340-143-04, 54340-143-05, 54340-144-01, 54340-144-02, 54340-144-03, 54340-145-01, 54340-145-02, 54340-145-03, 54340-146-01, 54340-146-02, 54340-146-03, 54340-146-04, 54340-147-01, 54340-147-02, 54340-147-03, 54340-148-01, 54340-148-02, 54340-148-03, 54340-164-07, 54340-164-08, 54340-165-08, 54340-165-09, 54340-166-09, 54340-167-01, 54340-167-02, 54340-167-03, 54340-190-02, 54340-190-03
- Packager: Henkel Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Purpose
- Uses
- Warnings
- When Using this Product
- Stop use and ask doctor if
- Keep out of reach of children.
- Directions
-
Inactive Ingredients
Lavendar Jasmine
Aqua (Water, Eau)· Lauramidopropylamine Oxide· Glycerin· Cetrimonium Chloride· Lauramine Oxide· Sodium Chloride· PEG-120 Methyl Glucose Dioleate · Citric Acid · Sodium Benzoate · Myristamidopropylamine Oxide · Parfum (Fragrance) · Zinc Sulfate • Dimethyl Lauramine • Tetrasodium EDTA ·Alcohol· Dimethyl Myristamine · Cl 17200 (Red 33) · Cl 42090 (Blue 1) ·
Lemon and Sage
Aqua (Water, Eau) · Lauramidopropylamine Oxide· Glycerin - Cetrimonium Chloride· Lauramine Oxide• Sodium Chloride - PEG-120 Methyl Glucose Dio!eate · Citric Acid - Sodium Benzoate · Myristamidopropylamine Oxide · Zinc Sulfate - Dimethyl Lauramine · Parfum (Fragrance) Tetrasodium EDTA ·Alcohol· Dimethyl Myristamine · Cl 19140 (Yellow 5) · Cl 47005 (Yellow 10)
Pomegranate Tangerine
Aqua (Water, Eau)· Lauramidopropylamine Oxide· Glycerin· Cetrimonium Chloride· Lauramine Oxide· Sodium Chloride· PEG-120 Methyl Glucose Dioleate · Citric Acid · Sodium Benzoate · Myristamidopropylamine Oxide · Parfum (Fragrance) · Zinc Sulfate · Dimethyl Lauramine · Tetrasodium EDTA ·Alcohol· Dimethyl Myristamine · Cl 15985 (Yellow 6) · Cl 17200 (Red 33) · Cl 16035 (Red 40)
Sweet Watermelon
Aqua (Water, Eau)· Lauramidopropylamine Oxide ·Glycerin· Cetrimonium Chloride· Lauramine Oxide· Sodium Chloride· PEG-120 Methyl Glucose Dioleate · Citric Acid · Sodium Benzoate · Parfum (Fragrance) · Myristamidopropylamine Oxide · Zinc Sulfate · Dimethyl Lauramine · Tetrasodium EDTA ·Alcohol· Dimethyl Myristamine · Cl 17200 (Red 33) · Cl 16035 (Red 40) · Cl 15985 (Yellow6)
Gold
Aqua (Water, Eau)· Lauramidopropylamine Oxide· Glycerin· Cetrimonium Chloride· Lauramine Oxide· Sodium Chloride·
PEG-120 Methyl Glucose Dioleate · Citric Acid · Sodium Benzoate · Myristamidopropylamlne Oxide · Zinc Sulfate ·
Parfum (Fragrance) • Dimethyl Lauramine • Tetrasodium EDTA ·Alcohol· Dimethyl Myrlstamine · Cl 19140 (Yellow 5) • Cl 14700 (Red 4)Aloe
Aqua (Water, Eau)• Lauramidopropylamine Oxide· Glycerin· Cetrimonium Chloride· Lauramine Oxide· Sodium Chloride·
PEG-120 Methyl Glucose Dioleate · Citric Acid · Sodium Benzoate · Myristamidopropylamine Oxide · Parfum (Fragrance)
· Zinc Sulfate · Aloe Barbadensis Leaf Juice· Dimethyl Lauramlne · Tetrasodium EDTA ·Alcohol· Dimethyl Myristamine
· Cl 19140 (Yellow 5) · Cl 42090 (Blue 1)Spring Water
Aqua (Water, Eau)· Lauramidopropylamine Oxide· Glycerin· Cetrimonium Chloride· Lauramine Oxide· Sodium Chloride·
PEG-120 Methyl Glucose Dioleate · Citric Acid · Sodium Benzoate · Myristamidopropylamine Oxide · Parfum (Fragrance) •
Zinc Sulfate • Dimethyl Lauramine • Tetrasodium EDTA ·Alcohol· Dimethyl Myristamine · Cl 42090 (Blue 1) · Cl 17200 (Red 33)White Tea
Aqua (Water, Eau) · Lauramidopropylamine Oxide · Glycerin · Lauramine Oxide · Cetrimonium Chloride·
Sodium Chloride· PEG-120 Methyl Glucose Dioleate · Citric Acid· Sodium Benzoate · Myristamidopropylam!ne Oxide·
Parfum (Fragrance)· Zinc Sulfate· Dimethyl Lauramine • Tetrasodium EDTA · Alcohol· Camellia Sinensis Leaf Extract·
Dimethyl Myristamine · Cl 42090 (Blue 1) · Cl 17200 (Red 33)Water Lilly
Aqua (Water, Eau) · Lauramidopropylamine Oxide · Glycerin · Lauramine Oxide · Cetrimonium Chloride·
Sodium Chloride· PEG-120 Methyl Glucose Dioleate · Citric Acid· Sodium Benzoate · Myristamidopropylam!ne Oxide·
Parfum (Fragrance)· Zinc Sulfate· Dimethyl Lauramine • Tetrasodium EDTA · Alcohol· Dimethyl Myristamine ·Fragrance Free
Aqua (Water, Eau) · Lauramidopropylamine Oxide · Glycerin · Lauramine Oxide · Cetrimonium Chloride·
Sodium Chloride· PEG-120 Methyl Glucose Dioleate · Citric Acid· Sodium Benzoate · Myristamidopropylam!ne Oxide·
· Zinc Sulfate· Dimethyl Lauramine • Tetrasodium EDTA · Alcohol· Dimethyl Myristamine ·Apple Blossom BW
Aqu a (Water,Eau ) • Cocamidopropyl Betaine • Lauramine Oxide • Cetrimonium Chloride • Sodium Chloride • Parfum (Fragrance) • Betaine • Citric Acid • PEG-150 Pentaer ythrityl Tetrastearate • Sod ium Benzoate • PPG-2 Hydrox yethyl Coca mide • PEG-2 0 0 Hydro gena te d Gl y c er y l Palma te • PEG-7 Glyceryl Cocoate • Dimethyl Lauramine • Tetrasodium EDTA • Dimethyl Myristamine • Alcohol • CI 17200 (Red 3)
Men's BW
Aqu a (Water,Eau ) • Cocamidopropyl Betaine • Lauramine Oxide • Cetrimonium Chloride • Sodium Chloride • Parfum (Fragrance) • Betaine • Citric Acid • PEG-150 Pentaer ythrityl Tetrastearate • Sod ium Benzoate • PPG-2 Hydrox yethyl Coca mide • PEG-2 0 0 Hydro gena te d Gl y c er y l Palma te • PEG-7 Glyceryl Cocoate • Dimethyl Lauramine • Tetrasodium EDTA • Dimethyl Myristamine • Alcohol • CI 17200 (Red 3)
- Questions
- Legal Entity
- ^Encountered in household settings
- Indications & Usage
- Topical Liquid
- Front and the back of the pack
-
INGREDIENTS AND APPEARANCE
DIAL COMPLETE LHS CLEAN AND GENTLE
liquid hand soap clean and gentle solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-165 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.35 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-165-08 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-165-09 325 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE LHS LAVENDER AND JASMINE
liquid hand soap lavender and jasmine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-141 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.35 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-141-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-141-02 325 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE ALOE
antibacterial hand wash aloe solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-146 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.27 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-146-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-146-02 277 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 3 NDC:54340-146-03 1180 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 4 NDC:54340-146-04 325 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE GOLD
antibacterial hand wash gold solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.43 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-143-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-143-02 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 3 NDC:54340-143-03 325 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 4 NDC:54340-143-04 946 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 5 NDC:54340-143-05 3780 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE SPRING WATER
antibacterial hand wash spring water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-142 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.34 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-142-02 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-142-04 946 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 3 NDC:54340-142-05 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 4 NDC:54340-142-03 325 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE WHITE TEA
antibacterial hand wash white tea solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-145 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.46 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-145-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-145-02 325 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 3 NDC:54340-145-03 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE LHS LEMON AND SAGE
liquid hand soap lemon and sage solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-147 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.08 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-147-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-147-02 325 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 3 NDC:54340-147-03 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE LHS SWEET WATERMELON
liquid hand soap sweet watermelon solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-144 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.35 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-144-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-144-02 325 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 3 NDC:54340-144-03 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE LHS POMEGRANATE AND TANGERINE
liquid hand soap pomegranate and tangerine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-148 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.35 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-148-01 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-148-02 325 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 3 NDC:54340-148-03 1530 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE LHS CLEAN AND GENTLE
liquid hand soap waterlilly solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-164 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.35 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-164-07 221 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 2 NDC:54340-164-08 325 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE BW MEN
dial complete bw men solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-166 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 90.35 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-166-09 473 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL COMPLETE BW APPLE BLOSSOM
dial complete bw apple blossom solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-167 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength D&C RED NO. 33 (UNII: 9DBA0SBB0L) 0.00008 g in 100 mL DIMETHYL LAURAMINE (UNII: 6V2OM30I1Z) 0.035 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.8 g in 100 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.4 g in 100 mL PEG-150 PENTAERYTHRITYL TETRASTEARATE (UNII: 8L4OOQ76AM) 0.45 g in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 7.6 g in 100 mL CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) 1.5 g in 100 mL WATER (UNII: 059QF0KO0R) 82.8 mL in 100 mL PEG-200 HYDROGENATED GLYCERYL PALMATE (UNII: W161T051Y1) 0.168 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 1.4 g in 100 mL LAURAMINE OXIDE (UNII: 4F6FC4MI8W) 2.1 g in 100 mL BETAINE (UNII: 3SCV180C9W) 0.85 g in 100 mL TETRASODIUM EDETATE DIHYDRATE (UNII: 3JGX4KKZ4A) 0.019 g in 100 mL PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) 0.042 g in 100 mL ALCOHOL (UNII: 3K9958V90M) 0.013 g in 100 mL PPG-2 HYDROXYETHYL COCAMIDE (UNII: 34N07GUJ3X) 0.25 g in 100 mL DIMETHYL MYRISTAMINE (UNII: 5E4O85D8T2) 0.014 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-167-01 473 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2020 01/01/2023 2 NDC:54340-167-02 473 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/31/2023 3 NDC:54340-167-03 621 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/31/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 DIAL ADVANCED CLEAN ANTIBACTERIAL BODY WASH APPLE BLOSSOM
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54340-190 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength D&C RED NO. 33 (UNII: 9DBA0SBB0L) 0.00008 g in 100 mL DIMETHYL LAURAMINE (UNII: 6V2OM30I1Z) 0.035 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.8 g in 100 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.4 g in 100 mL PEG-150 PENTAERYTHRITYL TETRASTEARATE (UNII: 8L4OOQ76AM) 0.45 g in 100 mL COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 7.6 g in 100 mL CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) 1.5 g in 100 mL WATER (UNII: 059QF0KO0R) 82.8 mL in 100 mL PEG-200 HYDROGENATED GLYCERYL PALMATE (UNII: W161T051Y1) 0.168 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 1.4 g in 100 mL LAURAMINE OXIDE (UNII: 4F6FC4MI8W) 2.1 g in 100 mL BETAINE (UNII: 3SCV180C9W) 0.85 g in 100 mL TETRASODIUM EDETATE DIHYDRATE (UNII: 3JGX4KKZ4A) 0.019 g in 100 mL PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) 0.042 g in 100 mL ALCOHOL (UNII: 3K9958V90M) 0.013 g in 100 mL PPG-2 HYDROXYETHYL COCAMIDE (UNII: 34N07GUJ3X) 0.25 g in 100 mL DIMETHYL MYRISTAMINE (UNII: 5E4O85D8T2) 0.014 g in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54340-190-02 473 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/31/2023 2 NDC:54340-190-03 621 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/31/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2022 Labeler - Henkel Corporation (080887708)