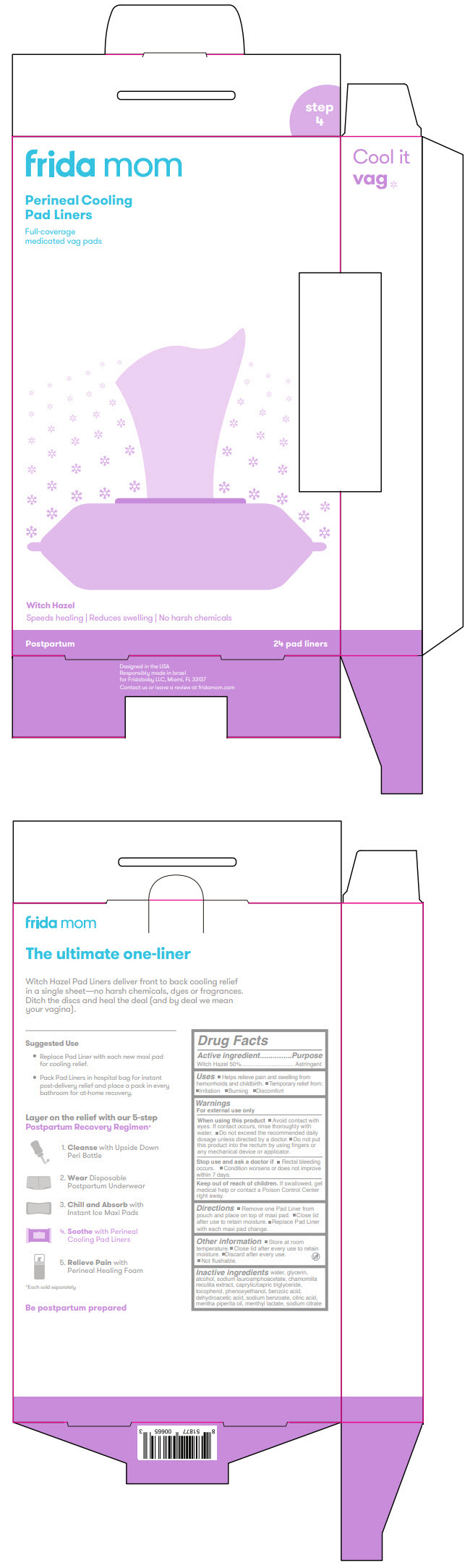
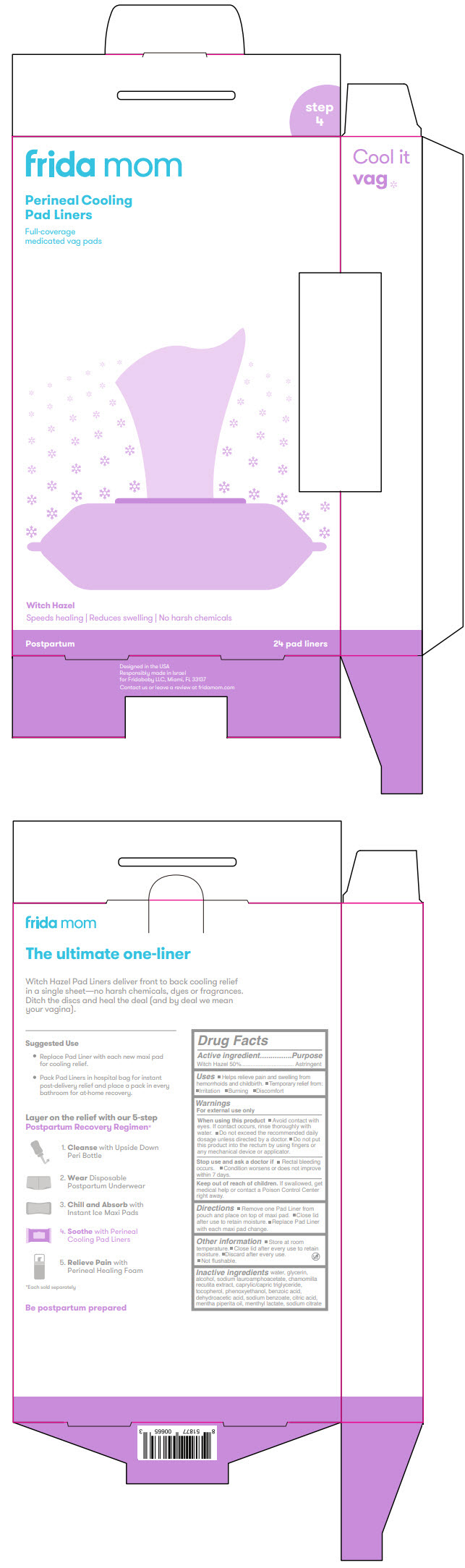
Label: FRIDA MOM PERINEAL COOLING PAD LINERS- witch hazel cloth
- NDC Code(s): 72705-100-01
- Packager: Fridababy, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- Avoid contact with eyes. If contact occurs, rinse thoroughly with water.
- Do not exceed the recommended daily dosage unless directed by a doctor.
- Do not put this product into the rectum by using fingers or any mechanical device or applicator.
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 24 Liner Pouch
-
INGREDIENTS AND APPEARANCE
FRIDA MOM PERINEAL COOLING PAD LINERS
witch hazel clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72705-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Witch Hazel (UNII: 101I4J0U34) (Witch Hazel - UNII:101I4J0U34) Witch Hazel 0.50 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) SODIUM LAUROAMPHOACETATE (UNII: SLK428451L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) BENZOIC ACID (UNII: 8SKN0B0MIM) DEHYDROACETIC ACID (UNII: 2KAG279R6R) SODIUM BENZOATE (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PEPPERMINT OIL (UNII: AV092KU4JH) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72705-100-01 24 in 1 POUCH; Type 0: Not a Combination Product 05/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 05/01/2019 Labeler - Fridababy, LLC (783729598) Establishment Name Address ID/FEI Business Operations Fischer Pharmaceuticals Ltd. 600410514 MANUFACTURE(72705-100)