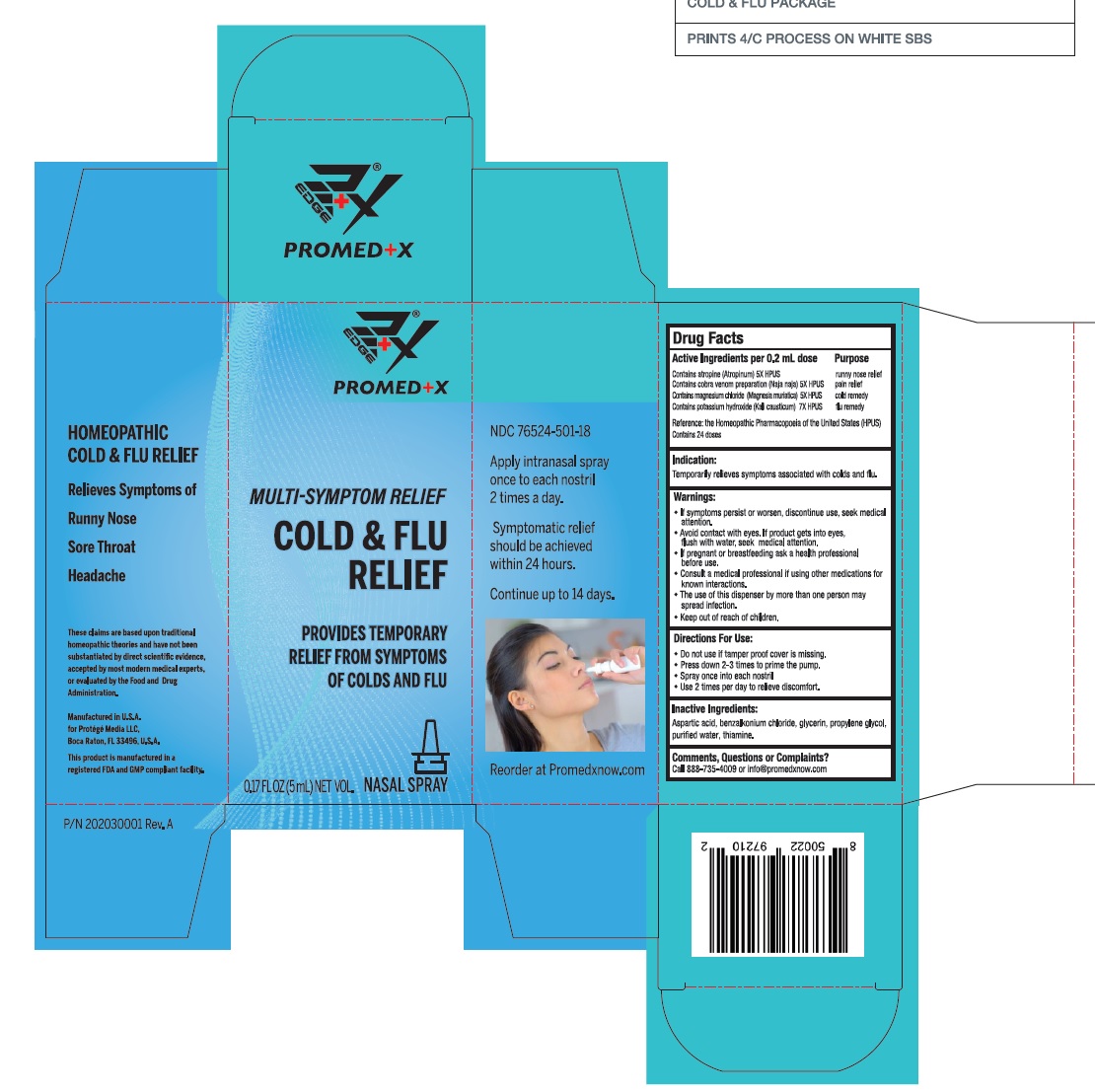
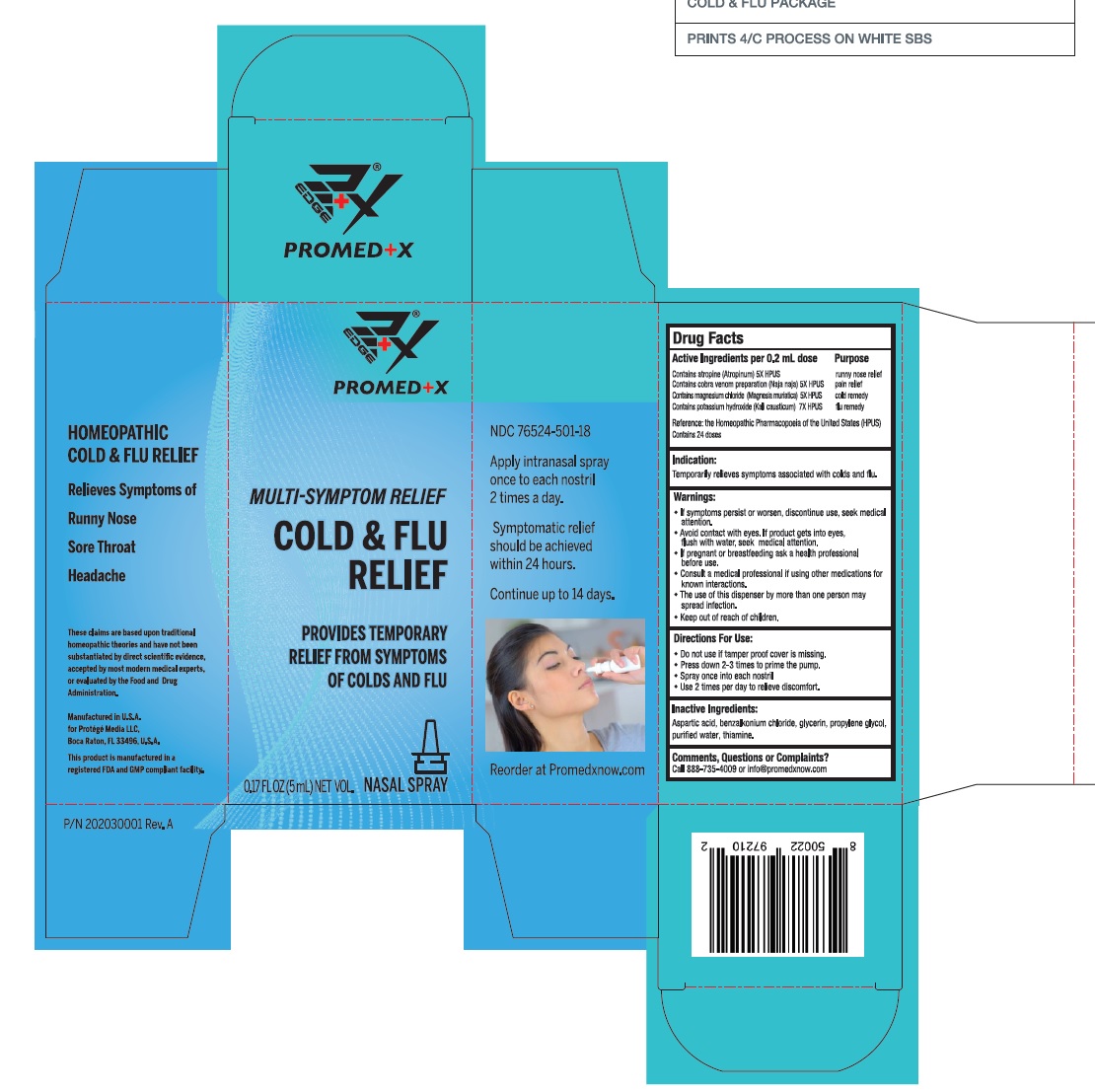
Label: COLD AND FLU RELIEF- atropine, naja naja venom, magnesium chloride, potassium hydroxide spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 76524-501-18 - Packager: PROTEGE' MEDIA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 13, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active ingredient Purpose
Contains atropine (Atropinum) 5X HPUS ………………………………….... runny nose relief
Contains cobra venom preparation (Naja naja) 5X HPUS ………..... pain relief
Contains magnesium chloride (Magnesia muriatica) 5X HPUS….... cold remedy
Contains potassium hydroxide (Kali causticum) 7X HPUS ………. .... flu remedy
Reference: the Homeopathic Pharmacopoeia of the United States (HPUS)Contains 24 doses
- PURPOSE
- Use
-
Warnings
If symptoms persist or worsen, discontinue use, seek medical attention.
• Avoid contact with eyes. If product gets into eyes, flush with water, seek medical attention.
• If pregnant or breastfeeding ask a health professional before use.
• Consult a medical professional if using other medications for known interactions.
• The use of this dispenser by more than one person may spread infection. - KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive ingredients
- Comments, Questions or Complaints?
- Product label
-
INGREDIENTS AND APPEARANCE
COLD AND FLU RELIEF
atropine, naja naja venom, magnesium chloride, potassium hydroxide sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76524-501 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPINE (UNII: 7C0697DR9I) (ATROPINE - UNII:7C0697DR9I) ATROPINE 5 [hp_X] in 0.2 mL NAJA NAJA VENOM (UNII: ZZ4AG7L7VM) (NAJA NAJA VENOM - UNII:ZZ4AG7L7VM) NAJA NAJA VENOM 5 [hp_X] in 0.2 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 5 [hp_X] in 0.2 mL POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) (HYDROXIDE ION - UNII:9159UV381P) POTASSIUM HYDROXIDE 7 [hp_X] in 0.2 mL Inactive Ingredients Ingredient Name Strength ASPARTIC ACID (UNII: 30KYC7MIAI) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) THIAMINE (UNII: X66NSO3N35) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76524-501-18 5 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 10/29/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/29/2020 Labeler - PROTEGE' MEDIA LLC (117063923)