Label: ADACEL TDAP (clostridium tetani toxoid antigen (formaldehyde inactivated), corynebacterium diphtheriae toxoid antigen (formaldehyde inactivated), bordetella pertussis toxoid antigen (glutaraldehyde inactivated), bordetella pertussis filamentous hemagglutinin antigen- formaldehyde inactivated, bordetella pertussis pertactin antigen, and bordetella pertussis fimbriae 2/3 antigen injection, suspension
-
NDC Code(s):
49281-400-05,
49281-400-10,
49281-400-20,
49281-400-58, view more49281-400-89
- Packager: Sanofi Pasteur Inc.
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated July 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Adacel safely and effectively. See full prescribing information for Adacel.
Adacel (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed), Suspension for Intramuscular Injection
Initial U.S. Approval: 2005INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
For intramuscular injection only.
- Each dose of Adacel is administered as a 0.5 mL injection. (2.1)
- For routine booster vaccination, a first dose of Adacel is administered 5 years or more after the last dose of Diphtheria and Tetanus Toxoids and Acellular Pertussis (DTaP) series or 5 years or more after vaccination with Tetanus and Diphtheria Toxoids Adsorbed (Td). A second dose of Adacel may be administered 8 years or more after the first dose with Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap).
- Adacel may be administered for tetanus prophylaxis for wound management. For management of a tetanus prone wound, a booster dose of Adacel may be administered if at least 5 years have elapsed since previous receipt of a tetanus toxoid containing vaccine. (2.2)
- To provide protection against pertussis in infants younger than 2 months of age, administer Adacel during the third trimester of pregnancy
DOSAGE FORMS AND STRENGTHS
- Single-dose vials and prefilled syringes containing a 0.5 mL suspension for injection. (3)
CONTRAINDICATIONS
- Severe allergic reaction (e.g., anaphylaxis) to any component of Adacel or any other diphtheria toxoid, tetanus toxoid and pertussis antigen-containing vaccine. (4.1)
- Encephalopathy (e.g., coma, decreased level of consciousness, prolonged seizures) within 7 days of administration of a previous pertussis antigen-containing vaccine. (4.2)
WARNINGS AND PRECAUTIONS
- If Guillain-Barré syndrome occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome may be increased following a subsequent dose of Adacel vaccine. (5.3)
- Progressive or unstable neurologic conditions are reasons to defer Adacel vaccination. (5.4)
- Persons who experienced an Arthus-type hypersensitivity reaction following a prior dose of a tetanus toxoid-containing vaccine should not receive Adacel unless at least 10 years have elapsed since the last dose of a tetanus toxoid-containing vaccine. (5.5)
- Syncope (fainting) can occur in association with administration of injectable vaccines, including Adacel. Procedures should be in place to prevent falling injury and manage syncopal reactions. (5.7)
ADVERSE REACTIONS
- Following the first vaccination with Adacel, the most common solicited adverse reactions within 0-14 days of vaccination for Adolescents (11-17 years of age)/Adults (18-64 years of age) were:
injection site pain (77.8%/65.7%), headache (43.7%/33.9%), body ache or muscle weakness (30.4%/21.9%), tiredness (30.2%/24.3%), injection site swelling (20.9%/21.0%), and injection site erythema (20.8%/24.7%). (6.1) - Following a second vaccination with Adacel, the most common solicited reactions occurring within 0-7 days of vaccination for Adults (18-64 years of age) were:
injection site pain (87.1%), myalgia (58.1%), headache (41.4%), malaise (33.3%), injection site swelling (6.9%), and injection site erythema (6.4%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pharmacovigilance Department, Sanofi Pasteur Inc., Discovery Drive, Swiftwater, PA 18370 at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 or http://vaers.hhs.gov.
DRUG INTERACTIONS
- When Adacel was administered concomitantly with trivalent inactivated influenza vaccine (TIV) to adults 19-64 years of age, a lower antibody response was observed for pertactin antigen as compared to Adacel administered alone. (7.1, 14.4)
- Immunosuppressive therapies may reduce the immune response to Adacel. (7.2)
- Do not mix Adacel with any other vaccine in the same syringe or vial.
USE IN SPECIFIC POPULATIONS
- Pregnancy Exposure Registry: contact Sanofi Pasteur Inc. at 1-800-822-2463 (1-800-VACCINE). (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Preparation for Administration
2.2 Administration, Dose and Schedule
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Encephalopathy
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
5.2 Guillain-Barré Syndrome and Brachial Neuritis
5.3 Progressive or Unstable Neurologic Disorders
5.4 Arthus-Type Hypersensitivity
5.5 Altered Immunocompetence
5.6 Syncope
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Concomitant Vaccine Administration
7.2 Immunosuppressive Treatments
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Immunological Evaluation in Adolescents and Adults, 11 through 64 Years of Age Following a First Vaccination with Adacel (Td506)
14.2 Immunological Evaluation in Adults, 18 through 64 Years of Age Following a Second Vaccination with Adacel
14.3 Study in Pregnant Women
14.4 Immune Responses to Pertussis Vaccination in Infants Born to Mothers Who Received Adacel During Pregnancy
14.5 Concomitant Hepatitis B Vaccine Administration
14.6 Concomitant Influenza Vaccine Administration
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
2.1 Preparation for Administration
Just before use, shake the vial or syringe well until a uniform, white, cloudy suspension results.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exist, the vaccine should not be administered.
Withdraw the 0.5 mL dose of vaccine from the single-dose vial using a sterile needle and syringe.
Adacel should not be combined through reconstitution or mixed with any other vaccine. Discard unused portion in vial.
2.2 Administration, Dose and Schedule
Adacel is administered as a single 0.5 mL intramuscular injection.
Routine Booster Vaccination
A first dose of Adacel is administered 5 years or more after the last dose of the Diphtheria and Tetanus Toxoids and Acellular Pertussis (DTaP) series or 5 years or more after a dose of Tetanus and Diphtheria Toxoids Adsorbed (Td). A second dose of Adacel may be administered 8 years or more after the first dose of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed (Tdap).
-
3 DOSAGE FORMS AND STRENGTHS
Adacel is a suspension for injection available in 0.5 mL single-dose vials and prefilled syringes. [See HOW SUPPLIED/STORAGE AND HANDLING (16).]
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
A severe allergic reaction (e.g., anaphylaxis) after a previous dose of any tetanus toxoid, diphtheria toxoid or pertussis containing vaccine or any other component of this vaccine is a contraindication to administration of Adacel. [See DESCRIPTION (11).] Because of uncertainty as to which component of the vaccine may be responsible, none of the components should be administered. Alternatively, such individuals may be referred to an allergist for evaluation if further immunizations are to be considered.
4.2 Encephalopathy
Encephalopathy (e.g., coma, prolonged seizures, or decreased level of consciousness) within 7 days of a previous dose of a pertussis containing vaccine not attributable to another identifiable cause is a contraindication to administration of any pertussis containing vaccine, including Adacel.
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Epinephrine hydrochloride solution (1:1,000) and other appropriate agents and equipment must be available for immediate use in case an anaphylactic or acute hypersensitivity reaction occurs.
5.2 Guillain-Barré Syndrome and Brachial Neuritis
If Guillain-Barré syndrome occurred within 6 weeks of receipt of prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome may be increased following a dose of Adacel. A review by the Institute of Medicine found evidence for acceptance of a causal relation between tetanus toxoid and brachial neuritis. (1)
5.3 Progressive or Unstable Neurologic Disorders
Progressive or unstable neurologic conditions are reasons to defer Adacel. It is not known whether administration of Adacel to persons with an unstable or progressive neurologic disorder might hasten manifestations of the disorder or affect the prognosis. Administration of Adacel to persons with an unstable or progressive neurologic disorder may result in diagnostic confusion between manifestations of the underlying illness and possible adverse effects of vaccination.
5.4 Arthus-Type Hypersensitivity
Persons who experienced an Arthus-type hypersensitivity reaction following a prior dose of a tetanus toxoid-containing vaccine should not receive Adacel unless at least 10 years have elapsed since the last dose of a tetanus toxoid containing vaccine.
5.5 Altered Immunocompetence
If Adacel is administered to immunocompromised persons, including persons receiving immunosuppressive therapy, the expected immune response may not be obtained. [See DRUG INTERACTIONS (7.2).]
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to vaccine use and for approximating rates of those events. As with any vaccine, there is the possibility that broad use of Adacel could reveal adverse reactions not observed in clinical trials.
The safety of a first vaccination with Adacel was evaluated in 5 clinical studies. Three of the studies were conducted in the U.S. and 2 were conducted in Canada. Of the study participants, 86% were Caucasian, 8% Black, 3% Hispanic, 1% Asian and 2% of other ethnic origin. A total of 7,143 individuals 10 through 64 years of age inclusive (4,695 adolescents 10 through 17 years of age and 2,448 adults 18 through 64 years of age) received a single dose of Adacel.
U.S. Adolescent and Adult Study of a First Vaccination with Adacel (Td506)
Clinical study Td506 was a randomized, observer-blind, active-controlled trial that enrolled adolescents 11 through 17 years of age (Adacel N = 1,184; DECAVAC (Tetanus and Diphtheria Toxoids Adsorbed; manufactured by Sanofi Pasteur Inc., Swiftwater, PA) N = 792) and adults 18 through 64 years of age (Adacel N = 1,752; DECAVAC N = 573). Study participants had not received tetanus or diphtheria-containing vaccines within the previous 5 years. Solicited local and systemic reactions and unsolicited adverse events were monitored daily for 14 days post vaccination using a diary card. From days 14 to 28 post vaccination, information on adverse events necessitating a medical contact, such as a telephone call, visit to an emergency room, physician's office or hospitalization, was obtained via telephone interview or at an interim clinic visit. From days 28 to 6 months post vaccination, participants were monitored for unexpected visits to a physician's office or to an emergency room, onset of serious illness, and hospitalizations. Information regarding adverse events that occurred in the 6-month post vaccination time period was obtained from participants via telephone contact. At least 96% of participants completed the 6-month follow-up evaluation.
The frequency of selected solicited adverse reactions (erythema, swelling, pain and fever) occurring during days 0 to 14 following vaccination with Adacel or Td vaccine in adolescents 11 through 17 years of age and adults 18 through 64 years of age are presented in Table 1. Most of these reactions were reported at a similar frequency in recipients of both Adacel and Td vaccine. Pain at the injection site was the most common adverse reaction in 62.9% to 77.8% of all vaccinees. In addition, overall rates of pain were higher in adolescent recipients of Adacel compared to Td vaccine recipients. Rates of moderate and severe pain in adolescents did not significantly differ between the Adacel and Td vaccine groups. Among adults, the rates of pain after receipt of Adacel or Td vaccine did not significantly differ. Fever of 38°C and higher was uncommon, although in the adolescent age group it occurred significantly more frequently in Adacel recipients than Td vaccine recipients.
Table 1: Frequencies of Solicited Injection Site Reactions and Fever for Adolescents and Adults, Days 0-14, Following a First Vaccination with Adacel or Td Vaccine in Study Td506 Adverse Reactions* Adolescents
11-17 years
Adacel
N† = 1,170-1,175
(%)Adolescents
11-17 years
Td‡
N† = 783-787 (%)Adults
18-64 years
Adacel
N† = 1,688-1,698
(%)Adults
18-64 years
Td‡
N† = 551-561
(%)- *
- The study sample size was designed to detect >10% differences between Adacel and Td vaccines for events of 'Any' intensity.
- †
- N = number of participants with available data.
- ‡
- Tetanus and Diphtheria Toxoids Adsorbed manufactured by Sanofi Pasteur Inc., Swiftwater, PA.
- §
- Adacel did not meet the non-inferiority criterion for rates of 'Any' Pain in adolescents compared to Td vaccine rates (upper limit of the 95% CI on the difference for Adacel minus Td vaccine was 10.7% whereas the criterion was <10%). For 'Any' Fever the non-inferiority criteria was met, however, 'Any' Fever was statistically higher in adolescents receiving Adacel.
- ¶
- Interfered with activities, but did not necessitate medical care or absenteeism.
- #
- Incapacitating, prevented the performance of usual activities, may have/or did necessitate medical care or absenteeism.
Injection Site Pain, Any 77.8§ 71.0 65.7 62.9 Injection Site Pain, Moderate¶ 18.0 15.6 15.1 10.2 Injection Site Pain, Severe# 1.5 0.6 1.1 0.9 Injection Site Swelling, Any 20.9 18.3 21.0 17.3 Injection Site Swelling, Moderate¶ 1.0 to 3.4 cm 6.5 5.7 7.6 5.4 Injection Site Swelling, Severe# ≥3.5 cm 6.4 5.5 5.8 5.5 Injection Site Swelling, Severe# ≥5 cm (2 inches) 2.8 3.6 3.2 2.7 Injection Site Swelling, Any 20.8 19.7 24.7 21.6 Injection Site Swelling, Moderate¶1.0 to 3.4 cm 5.9 4.6 8.0 8.4 Injection Site Swelling, Severe#≥3.5 cm 6.0 5.3 6.2 4.8 Injection Site Swelling, Severe#≥5 cm (2 inches) 2.7 2.9 4.0 3.0 Fever, ≥38.0°C (≥100.4°F) 5.0§ 2.7 1.4 1.1 Fever, ≥38.8°C to ≤39.4°C
(≥102.0°F to ≤103.0°F)0.9 0.6 0.4 0.2 Fever, ≥39.5°C (≥103.1°F) 0.2 0.1 0.0 0.2 The frequency of other solicited adverse reactions (days 0-14) are presented in Table 2. The rates of these reactions following a first vaccination with Adacel were comparable with those observed with Td vaccine. Headache was the most frequent systemic reaction and was usually of mild to moderate intensity.
Table 2: Frequencies of Other Solicited Adverse Reactions for Adolescents and Adults, Days 0-14, Following a First Vaccination with Adacel or Td Vaccine in Study Td506 Adverse Reaction Adolescents 11-17 years
Adacel
N* = 1,174-1,175
(%)Adolescents 11-17 years
Td†
N* = 787
(%)Adults 18-64 years
Adacel
N* = 1,697-1,698
(%)Adults 18-64 years
Td†
N* = 560-561
(%)- *
- N = number of participants with available data.
- †
- Tetanus and Diphtheria Toxoids Adsorbed manufactured by Sanofi Pasteur Inc., Swiftwater, PA.
- ‡
- Interfered with activities, but did not necessitate medical care or absenteeism.
- §
- Incapacitating, prevented the performance of usual activities, may have/or did necessitate medical care or absenteeism.
Headache, Any 43.7 40.4 33.9 34.1 Headache, Moderate‡ 14.2 11.1 11.4 10.5 Headache, Severe§ 2.0 1.5 2.8 2.1 Body Ache or Muscle Weakness, Any 30.4 29.9 21.9 18.8 Body Ache or Muscle Weakness, Moderate‡ 8.5 6.9 6.1 5.7 Body Ache or Muscle Weakness, Severe§ 1.3 0.9 1.2 0.9 Tiredness, Any 30.2 27.3 24.3 20.7 Tiredness, Moderate‡ 9.8 7.5 6.9 6.1 Tiredness, Severe§ 1.2 1.0 1.3 0.5 Chills, Any 15.1 12.6 8.1 6.6 Chills, Moderate‡ 3.2 2.5 1.3 1.6 Chills, Severe§ 0.5 0.1 0.7 0.5 Sore and Swollen Joints, Any 11.3 11.7 9.1 7.0 Sore and Swollen Joints, Moderate‡ 2.6 2.5 2.5 2.1 Sore and Swollen Joints, Severe§ 0.3 0.1 0.5 0.5 Nausea, Any 13.3 12.3 9.2 7.9 Nausea, Moderate‡ 3.2 3.2 2.5 1.8 Nausea, Severe§ 1.0 0.6 0.8 0.5 Lymph Node Swelling, Any 6.6 5.3 6.5 4.1 Lymph Node Swelling, Moderate‡ 1.0 0.5 1.2 0.5 Lymph Node Swelling, Severe§ 0.1 0.0 0.1 0.0 Diarrhea, Any 10.3 10.2 10.3 11.3 Diarrhea, Moderate‡ 1.9 2.0 2.2 2.7 Diarrhea, Severe§ 0.3 0.0 0.5 0.5 Vomiting, Any 4.6 2.8 3.0 1.8 Vomiting, Moderate‡ 1.2 1.1 1.0 0.9 Vomiting, Severe§ 0.5 0.3 0.5 0.2 Rash, Any 2.7 2.0 2.0 2.3 Injection site and systemic solicited reactions occurred at similar rates in Adacel and Td vaccine recipients in the 3 day post-vaccination period. Most injection site reactions occurred within the first 3 days after vaccination (with a mean duration of less than 3 days). The rates of unsolicited adverse events reported from days 14-28 post-vaccination were comparable between the two vaccine groups, as were the rates of unsolicited adverse events from day 28 through 6 months. There were no spontaneous reports of extensive limb swelling of the injected limb in study Td506, nor in the other three studies which also contributed to the safety database for Adacel.
Adult Study of a Second Vaccination with Adacel (NCT01439165)
In a randomized, observer-blind, active-controlled, multi-center study (NCT01439165), adults 18 through 64 years of age who had received a first dose of Adacel 8-12 years previously were enrolled and randomized to receive either Adacel (N = 1002) or a US licensed Td vaccine, TENIVAC (Tetanus and Diphtheria Toxoids Adsorbed; manufactured by Sanofi Pasteur, Limited) (N = 328). Subjects were recruited from the primary licensure study Td506 and the Canadian general public and had not received Td or Tdap vaccine since their initial Adacel dose. The demographic characteristics for study participants were similar for both vaccine groups. The mean ages were 28.9 years for the Adacel group and 29.2 years for the Td group. Overall, there were more female participants in both the Adacel group and Td group; 64.5% and 64.6%, respectively. In both vaccine groups, greater than 94% of subjects identified as white and 99% as non-Hispanic or Latino.
Safety data were collected from all participants who received the study vaccine (N = 999 for the Adacel group; N = 328 for the Td group). Solicited local and systemic reactions and unsolicited adverse events were monitored for 7 days post-vaccination using a diary card. Unsolicited adverse events were collected for approximately 28 days post-vaccination. Serious adverse events were collected throughout the study period (up to 6 months post-vaccination).
Solicited adverse reactions reported to occur during days 0-7 following vaccination are presented in Table 3.
Table 3: Frequencies of Solicited Adverse Reactions 0-7 Days Following a Second Vaccination with Adacel Compared to Td Vaccine in Study NCT01439165 - Safety Analysis Set Adverse Reaction Adacel
(N=999)
(%)Td Adsorbed*
(N=328)
(%)N = number of participants with available data Injection site pain, Any 87.1 87.4 Injection site pain, Grade 2† 28.5 31.4 Injection site pain, Grade 3‡ 3.6 2.8 Injection site erythema, Any 6.4 5.5 Injection site erythema, Grade 2 (≥51 to ≤100 mm) 2.1 2.8 Injection site erythema, Grade 3 (˃100 mm) 0.2 0.0 Injection site swelling, Any 6.9 8.0 Injection site swelling, Grade 2 (≥51 to ≤100 mm) 2.4 2.2 Injection site swelling, Grade 3 (˃100 mm) 0.3 0.0 Fever, Any 0.9 1.8 Fever, Grade 2 (≥38.5°C to ≤38.9°C or ≥101.2°F to ≤102.0°F 0.3 0.6 Fever, Grade 3 (≥102.1°F) 0.2 0.3 Headache, Any 41.4 39.1 Headache, Grade 2† 12.4 10.5 Headache, Grade 3‡ 2.6 4.0 Malaise, Any 33.3 30.8 Malaise, Grade 2† 9.3 9.8 Malaise, Grade 3‡ 3.0 3.7 Myalgia, Any 58.1 58.2 Myalgia, Grade 2† 18.7 16.9 Myalgia, Grade 3‡ 3.0 3.1 Adult Study of a Second Vaccination with Adacel (NCT00347958)
Study NCT00347958 was a descriptive, open-label, post-marketing, multi-center study evaluating the safety of Adacel readministration in adults 5 years following a previous dose of Adacel. The mean age of subjects was 31.7 years, there were more females (52.2%) than males (47.8%) and 89.9% of subjects were Caucasian. Solicited adverse reactions were collected for 14 days following vaccination. SAEs were monitored for 6 months following vaccination. A total of 545 subjects 16-69 years of age were enrolled. All participants in this study received a first dose of Adacel vaccine as part of Sanofi Pasteur studies Td501, Td502, or Td505. Approximately 90% of the participants had at least one solicited injection site reaction. The most frequently reported injection site reactions were pain in 87.6% of subjects, followed by erythema/redness in 28.6%, and swelling in 25.6%. Approximately 77% of the participants had at least one solicited systemic reaction. The most frequently reported solicited systemic adverse reactions in subjects who received a second dose of Adacel were myalgia (61%), followed by headache (53.2%), malaise (38.2%), and fever (6.5%).
Injection Site and Systemic Reactions Following Adacel Given Concomitantly with Hepatitis B Vaccine
In the concomitant vaccination study with Adacel (first vaccination) and Hepatitis B vaccine [Recombivax HB] (Td501) [See CLINICAL STUDIES (14)], injection site and systemic adverse events were monitored daily for 14 days post-vaccination using a diary card. Injection site adverse events were only monitored at site/arm of Adacel administration. Unsolicited reactions (including immediate reactions, serious adverse events and events that elicited seeking medical attention) were collected at a clinic visit or via telephone interview for the duration of the trial, i.e., up to 6 months post-vaccination.
The rates reported for fever and injection site pain (at the Adacel administration site) were similar when Adacel and Hepatitis B vaccine were given concurrently or separately. However, the rates of injection site erythema (23.4% for concomitant vaccination and 21.4% for separate administration) and swelling (23.9% for concomitant vaccination and 17.9% for separate administration) at the Adacel administration site were increased when coadministered. Swollen and/or sore joints were reported by 22.5% for concomitant vaccination and 17.9% for separate administration. The rates of generalized body aches in the individuals who reported swollen and/or sore joints were 86.7% for concomitant vaccination and 72.2% for separate administration. Most joint complaints were mild in intensity with a mean duration of 1.8 days. The incidence of other solicited and unsolicited adverse events were not different between the 2 study groups.
Injection Site and Systemic Reactions Following Adacel Given Concomitantly with Trivalent Inactivated Influenza Vaccine (TIV)
In the concomitant vaccination study with Adacel (first vaccination) and trivalent inactivated influenza vaccine [Fluzone] (Td502) [See CLINICAL STUDIES (14)], injection site and systemic adverse events were monitored for 14 days post-vaccination using a diary card. All unsolicited reactions occurring through day 14 were collected. From day 14 to the end of the trial, i.e., up to 84 days, only events that elicited seeking medical attention were collected.
The rates of fever and injection site erythema and swelling were similar for recipients of concurrent and separate administration of Adacel and TIV. However, pain at the Adacel injection site occurred at statistically higher rates following concurrent administration (66.6%) versus separate administration (60.8%). The rates of sore and/or swollen joints were 13% for concurrent administration and 9% for separate administration. Most joint complaints were mild in intensity with a mean duration of 2.0 days. The incidence of other solicited and unsolicited adverse events was similar between the 2 study groups.
Additional Studies
In an additional study (Td505), 1,806 adolescents 11 through 17 years of age received Adacel (first vaccination) as part of the lot consistency study used to support Adacel licensure. This study was a randomized, double-blind, multi-center trial designed to assess lot consistency as measured by the safety and immunogenicity of 3 lots of Adacel when given as a booster dose to adolescents 11 through 17 years of age inclusive. Local and systemic adverse events were monitored for 14 days post-vaccination using a diary card. Unsolicited adverse events and serious adverse events were collected for 28 days post-vaccination. Pain was the most frequently reported local adverse event occurring in approximately 80% of all participants. Headache was the most frequently reported systemic event occurring in approximately 44% of all participants. Sore and/or swollen joints were reported by approximately 14% of participants. Most joint complaints were mild in intensity with a mean duration of 2.0 days.
An additional 962 adolescents and adults received Adacel in three supportive Canadian studies (TC9704, Td9707 and TD9805) used as the basis for licensure in other countries. Within these clinical trials, the rates of local and systemic reactions following the first vaccination with Adacel were similar to those reported in the four principal trials in the U.S. with the exception of a higher rate (86%) of adults experiencing "any" local injection site pain. The rate of severe pain (0.8%), however, was comparable to the rates reported in four principal trials conducted in the US. There was one spontaneous report of whole-arm swelling of the injected limb among the 277 Td vaccine recipients, and two spontaneous reports among the 962 Adacel recipients in the supportive Canadian studies.
An additional study (NCT01311557) enrolled 1,302 individuals in an open label, two-arm, multicenter trial (651 participants in each group) to evaluate the safety and immunogenicity of a first vaccination with Adacel administered to persons 10 to <11 years of age compared to persons 11 to <12 years of age. Immediate reactions were monitored for 20 minutes post-vaccination. Solicited local and systemic adverse events were monitored for 7 days post-vaccination using a diary card. Unsolicited and serious adverse events were collected for approximately 30 days post-vaccination. Similar rates of immediate, solicited and unsolicited adverse reactions were reported in each of the two age cohorts. One serious adverse event, not related to vaccination, was reported in the younger age group.
Serious Adverse Events
Throughout the 6-month follow-up period following a first vaccination with Adacel in study Td506, SAEs were reported in 1.5% of Adacel recipients and in 1.4% of Td vaccine recipients. Two SAEs in adults were neuropathic events that occurred within 28 days of Adacel administration; one severe migraine with unilateral facial paralysis and one diagnosis of nerve compression in neck and left arm. Similar or lower rates of serious adverse events were reported in the other trials following a first vaccination with Adacel in participants up to 64 years of age and no additional neuropathic events were reported.
In study NCT01439165 when a second vaccination of Adacel was administered 8-12 years following the initial vaccination of Adacel, a total of 8 participants (0.8%) in the Adacel group and 1 participant (0.3%) in the Td group reported SAEs during the 6-month follow-up period. All SAEs were considered by the investigator to be unrelated to the study vaccine.
In study NCT00347958, seven participants experienced an SAE, all of which were considered by the investigator to be unrelated to the study vaccine.
6.2 Postmarketing Experience
The following adverse events of Adacel have been spontaneously reported in the US and other countries. Because these events are reported voluntarily from a population of uncertain size, it may not be possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
The following adverse events were included based on one or more of the following factors: severity, frequency of reporting, or strength of evidence for a causal relationship to Adacel.
-
Immune system disorders
Anaphylactic reaction, hypersensitivity reaction (angioedema, edema, rash, hypotension) -
Nervous system disorders
Paresthesia, hypoesthesia, Guillain-Barré syndrome, brachial neuritis, facial palsy, convulsion, syncope, myelitis -
Cardiac disorders
Myocarditis -
Skin and subcutaneous tissue disorders
Pruritus, urticaria -
Musculoskeletal and connective tissue disorders
Myositis, muscle spasm -
General disorders and administration site conditions
Large injection site reactions (>50 mm), extensive limb swelling from the injection site beyond one or both joints
Injection site bruising, injection site nodule, sterile abscess, Arthus hypersensitivity
-
7 DRUG INTERACTIONS
7.1 Concomitant Vaccine Administration
When Adacel is administered concomitantly with other injectable vaccines or Tetanus Immune Globulin, they should be given with separate syringes and at different injection sites. Adacel should not be mixed with any other vaccine in the same syringe or vial.
Trivalent Inactivated Influenza Vaccine (TIV)
In a clinical study Adacel (first vaccination) was administered concomitantly with a US-licensed trivalent inactivated influenza vaccine (TIV). [See ADVERSE REACTIONS (6.1) and CLINICAL STUDIES (14).]
No interference in tetanus and diphtheria seroprotection rates and responses to influenza vaccine, detoxified pertussis toxin (PT), fimbriae types 2 and 3 (FIM) or filamentous hemagglutinin (FHA) were observed when Adacel vaccine was administered concomitantly with TIV compared to separate administration. A lower pertactin (PRN) GMC was observed when Adacel was administered concomitantly with TIV compared to separate administration.
7.2 Immunosuppressive Treatments
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs and corticosteroids (used in greater than physiologic doses), may reduce the immune response to vaccines. [See WARNINGS AND PRECAUTIONS (5.6).]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Adacel during pregnancy. Women who receive Adacel during pregnancy are encouraged to contact directly, or have their healthcare professional contact, Sanofi Pasteur Inc. at 1-800-822-2463 (1-800-VACCINE) or online at https://www.sanofipasteurpregnancyregistry.com.
Risk Summary
All pregnancies have a risk of birth defect, loss or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Available data suggest the rates of major birth defects and miscarriage in women who receive Adacel within 30 days prior to pregnancy or during pregnancy are consistent with estimated background rates. (See Human Data)
Two developmental toxicity studies were performed in female rabbits given 0.5 mL (a single human dose) of Adacel twice prior and during gestation. The studies revealed no evidence of harm to the fetus due to Adacel. (See Animal Data)
Data
Human Data
A retrospective passive surveillance study (NCT00258882) included women who received Adacel during pregnancy (n=225) and controls (n=675) matched by age and date of first positive pregnancy test. Of the 225 Adacel recipients, 39 women received Adacel within two weeks prior to the date of their last menstrual period (LMP), 110 were vaccinated during the 1st trimester (< 12 weeks gestation), 33 during the 2nd trimester (12 to < 27 weeks gestation), 14 in 3rd trimester (11 from 27 to <36 weeks gestation and 3 from ≥36 weeks gestation), and 29 were unknown. There were 21 reports of spontaneous abortion (9.3%) and 15 congenital anomalies (6.7%) in the Adacel exposed group, and 102 spontaneous abortions (15%) and 57 congenital anomalies (8.4%) in the control group.
An assessment of data from the ongoing pregnancy registry included 1236 prospective reports of exposure to Adacel during pregnancy since 10 June 2005 through 16 October 2022. Among the 286 reports with known pregnancy outcomes, 118 women were exposed to Adacel in the first trimester with 1 congenital anomaly and 14 spontaneous abortions; 54 women were exposed to Adacel in the second trimester with one congenital anomaly; 114 women were exposed to Adacel in the third trimester with 2 congenital anomalies; 76 women were exposed to Adacel at an unknown trimester with 1 congenital anomaly. The rates of assessed outcomes in the prospective population were consistent with estimated background rates.
Animal Data
The effect of Adacel on embryo-fetal and pre-weaning development was evaluated in two developmental toxicity studies in female rabbits. Animals were administered 0.5 mL (a single human dose) of Adacel twice prior to gestation, during the period of organogenesis (gestation day 6) and later during pregnancy on gestation day 29. No adverse effects on pregnancy, parturition, lactation, embryo-fetal or pre-weaning development were observed. There were no vaccine related fetal malformations or other evidence of teratogenesis noted in this study.
8.2 Lactation
Risk Summary
It is not known whether Adacel vaccine components are excreted in human milk. Data are not available to assess the effect of administration of Adacel on breast-fed infants or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Adacel and any potential adverse effects on the breastfed child from Adacel or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
Adacel is not approved for individuals less than 10 years of age. Safety and effectiveness of Adacel in persons less than 10 years of age in the U.S. have not been established.
8.5 Geriatric Use
Adacel is not approved for use in individuals 65 years of age and older.
In a clinical study, individuals 65 years of age and older received a single dose of Adacel. Based on prespecified criteria, persons 65 years of age and older who received a dose of Adacel had lower geometric mean concentrations of antibodies to PT, PRN and FIM when compared to infants who had received a primary series of DAPTACEL®, Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed (DTaP). [See CLINICAL STUDIES (14) for description of DAPTACEL.]
-
11 DESCRIPTION
Adacel is a sterile isotonic suspension of tetanus and diphtheria toxoids and pertussis antigens adsorbed on aluminum phosphate, for intramuscular injection.
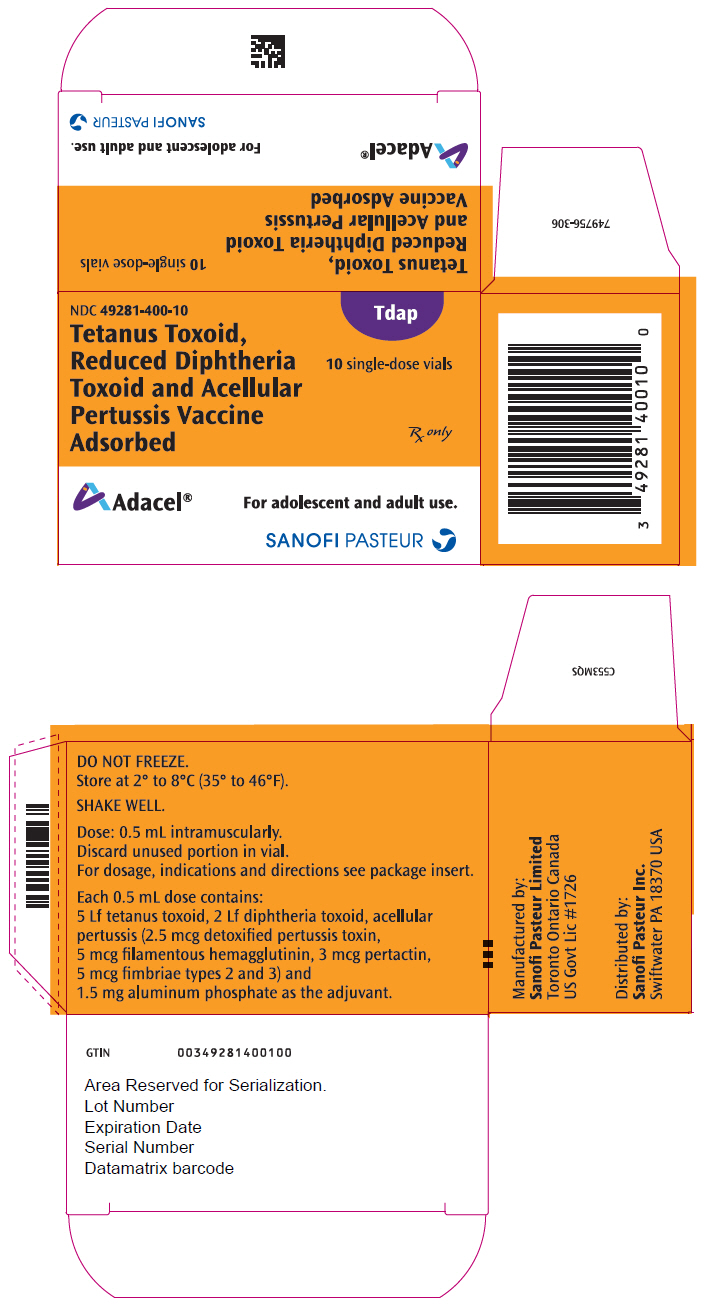
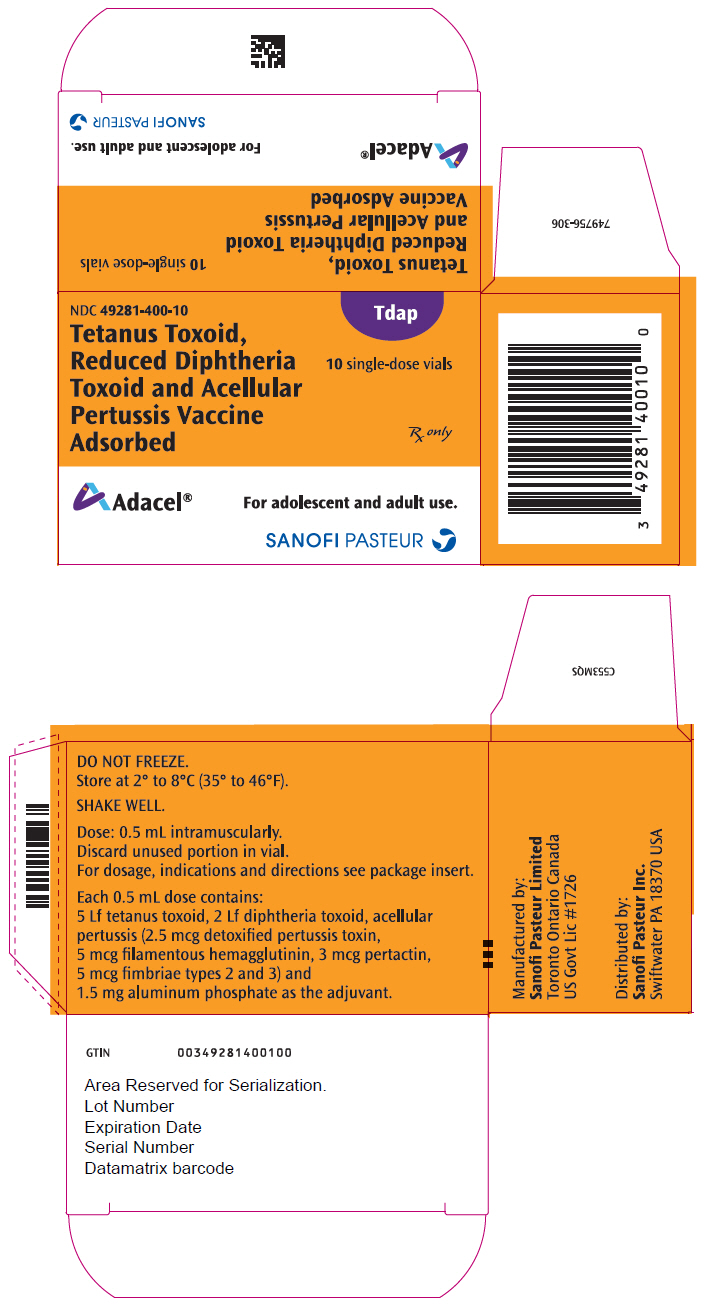
Each 0.5 mL dose contains 5 Lf tetanus toxoid (T), 2 Lf diphtheria toxoid (d), and acellular pertussis antigens [2.5 mcg detoxified pertussis toxin (PT), 5 mcg filamentous hemagglutinin (FHA), 3 mcg pertactin (PRN), 5 mcg fimbriae types 2 and 3 (FIM)]. Other ingredients per 0.5 mL dose include 1.5 mg aluminum phosphate (0.33 mg aluminum) as the adjuvant, ≤5 mcg residual formaldehyde, <50 ng residual glutaraldehyde and 3.3 mg (0.6% v/v) 2-phenoxyethanol (not as a preservative). The antigens are the same as those in DAPTACEL; however, Adacel is formulated with reduced quantities of diphtheria and detoxified PT.
The acellular pertussis vaccine components are produced from Bordetella pertussis cultures grown in Stainer-Scholte medium (2) modified by the addition of casamino acids and dimethyl-beta-cyclodextrin. PT, FHA and PRN are isolated separately from the supernatant culture medium. FIM are extracted and copurified from the bacterial cells. The pertussis antigens are purified by sequential filtration, salt-precipitation, ultrafiltration and chromatography. PT is detoxified with glutaraldehyde, FHA is treated with formaldehyde, and the residual aldehydes are removed by ultrafiltration. The individual antigens are adsorbed onto aluminum phosphate.
The tetanus toxin is produced from Clostridium tetani grown in modified Mueller-Miller casamino acid medium without beef heart infusion. (3) Tetanus toxin is detoxified with formaldehyde and purified by ammonium sulfate fractionation and diafiltration. Corynebacterium diphtheriae is grown in modified Mueller's growth medium. (4) After purification by ammonium sulfate fractionation, diphtheria toxin is detoxified with formaldehyde and diafiltered.
The adsorbed diphtheria, tetanus and acellular pertussis components are combined with aluminum phosphate (as adjuvant), 2-phenoxyethanol (not as a preservative) and water for injection. Adacel does not contain a preservative.
In the guinea pig potency test, the tetanus component induces at least 2 neutralizing units/mL of serum and the diphtheria component induces at least 0.5 neutralizing units/mL of serum. The potency of the acellular pertussis vaccine components is evaluated by the antibody response of immunized mice to detoxified PT, FHA, PRN and FIM as measured by enzyme-linked immunosorbent assay (ELISA).
Diphtheria and tetanus toxoids are individually adsorbed onto aluminum phosphate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tetanus
Tetanus is a disease manifested primarily by neuromuscular dysfunction caused by a potent exotoxin released by C tetani.
Protection against disease is due to the development of neutralizing antibodies to tetanus toxin. A serum tetanus antitoxin level of at least 0.01 IU/mL, measured by neutralization assay is considered the minimum protective level. (5) (6)
Diphtheria
Diphtheria is an acute toxin-mediated disease caused by toxigenic strains of C diphtheriae. Protection against disease is due to the development of neutralizing antibodies to diphtheria toxin. A serum diphtheria antitoxin level of 0.01 IU/mL is the lowest level giving some degree of protection. Antitoxin levels of at least 0.1 IU/mL are generally regarded as protective. (5) Levels of 1.0 IU/mL have been associated with long-term protection. (7)
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The effectiveness of the tetanus toxoid and diphtheria toxoid used in Adacel was based on the immune response to these antigens compared to a US licensed Tetanus and Diphtheria Toxoids Adsorbed For Adult Use (Td) vaccine manufactured by Sanofi Pasteur Inc., Swiftwater, PA. The primary measures for immune response to the diphtheria and tetanus toxoids were the percentage of participants attaining an antibody level of at least 0.1 IU/mL.
The effectiveness of the pertussis antigens used in Adacel was evaluated based on a comparison of pertussis antibody levels achieved in recipients of Adacel with those obtained in infants after three or four doses of DAPTACEL. For the first dose of Adacel, the comparisons were to infants who received three doses of DAPTACEL in the Sweden I Efficacy trial. For the second dose of Adacel, for the evaluation of FHA, PRN, and FIM antibody levels, the comparisons were to infants who received three doses of DAPTACEL in the Sweden I Efficacy trial; for evaluation of PT antibody levels, the comparison was to infants who received four doses of DAPTACEL in a US safety and immunogenicity study (Study NCT00255047). In the Sweden I Efficacy Trial, three doses of DAPTACEL vaccine were shown to confer a protective efficacy of 84.9% (95% CI: 80.1%, 88.6%) against WHO defined pertussis (21 days of paroxysmal cough with laboratory-confirmed B pertussis infection or epidemiological link to a confirmed case). The protective efficacy against mild pertussis (defined as at least one day of cough with laboratory-confirmed B pertussis infection) was 77.9% (95% CI: 72.6%, 82.2%). (8)
In addition, the ability of Adacel to elicit a booster response (defined as rise in antibody concentration after vaccination) to the tetanus, diphtheria and pertussis antigens following vaccination was evaluated.
14.1 Immunological Evaluation in Adolescents and Adults, 11 through 64 Years of Age Following a First Vaccination with Adacel (Td506)
Study Td506 was a comparative, multi-center, randomized, observer-blind, controlled trial which enrolled 4,480 participants; 2,053 adolescents (11-17 years of age) and 2,427 adults (18-64 years of age). Enrollment was stratified by age to ensure adequate representation across the entire age range. Participants had not received a tetanus or diphtheria toxoid containing vaccine within the previous 5 years. After enrollment participants were randomized to receive one dose of either Adacel or Td vaccine. A total of 4,461 randomized participants were vaccinated. The per-protocol immunogenicity subset included 1,270 Adacel recipients and 1,026 Td vaccine recipients. Sera were obtained before and approximately 35 days after vaccination. [Blinding procedures for safety assessments are described in ADVERSE REACTIONS (6).]
Demographic characteristics were similar within age groups and between the vaccine groups. A total of 76% of the adolescents and 1.1% of the adults reported a history of receiving 5 previous doses of diphtheria-tetanus-pertussis containing vaccines. Anti-tetanus and anti-diphtheria seroprotection rates (≥0.1 IU/mL) and booster response rates were comparable between Adacel and Td vaccines. (See Table 4 and Table 5.) Adacel induced pertussis antibody levels that were non-inferior to those of Swedish infants who received three doses of DAPTACEL vaccine (Sweden I Efficacy Study). (See Table 6.) Acceptable booster responses to each of the pertussis antigens were also demonstrated, i.e., the percentage of participants with a booster response exceeded the predefined lower limit. (See Table 7.)
Table 4: Pre-vaccination and Post-vaccination Antibody Responses and Booster Response Rates to Tetanus Toxoid Following a First Vaccination with Adacel Vaccine as Compared to Td Vaccine in Adolescents and Adults 11 through 64 Years of Age (Td506) Anti-Tetanus toxoid (IU/mL)
Pre-vaccinationAnti-Tetanus toxoid (IU/mL)
Pre-vaccinationAnti-Tetanus toxoid (IU/mL)
1 Month Post-vaccinationAnti-Tetanus toxoid (IU/mL)
1 Month Post-vaccinationAnti-Tetanus toxoid (IU/mL)
1 Month Post-vaccinationAge Group
(years)Vaccine N* % ≥0.10
(95% CI)% ≥1.0
(95% CI)% ≥0.10
(95% CI)% ≥1.0
(95% CI)% Booster†
(95% CI)- *
- N = number of participants in the per-protocol population with available data.
- †
- Booster response is defined as: A 4-fold rise in antibody concentration, if the pre-vaccination concentration was equal to or below the cut-off value and a 2-fold rise in antibody concentration if the pre-vaccination concentration was above the cut-off value. The cut-off value for tetanus was 2.7 IU/mL.
- ‡
- Seroprotection rates at ≥0.10 IU/mL and booster response rates to Adacel were non-inferior to Td vaccine (upper limit of the 95% CI on the difference for Td vaccine minus Adacel <10%).
- §
- Seroprotection rates at ≥1.0 IU/mL were not prospectively defined as a primary endpoint.
- ¶
- Tetanus and Diphtheria Toxoids Adsorbed manufactured by Sanofi Pasteur Inc., Swiftwater, PA.
11-17 Adacel 527 99.6
(98.6, 100.0)44.6
(40.3, 49.0)100.0‡
(99.3, 100.0)99.6§
(98.6, 100.0)91.7‡
(89.0, 93.9)11-17 Td¶ 516 99.2
(98.0, 99.8)43.8
(39.5, 48.2)100.0
(99.3, 100.0)99.4
(98.3, 99.9)91.3
(88.5, 93.6)18-64 Adacel 742-743 97.3
(95.9, 98.3)72.9
(69.6, 76.1)100.0‡
(99.5, 100.0)97.8§
(96.5, 98.8)63.1‡
(59.5, 66.6)18-64 Td¶ 509 95.9
(93.8, 97.4)70.3
(66.2, 74.3)99.8
(98.9, 100.0)98.2
(96.7, 99.2)66.8
(62.5, 70.9)Table 5: Pre-vaccination and Post-vaccination Antibody Responses and Booster Response Rates to Diphtheria Toxoid Following a First Vaccination with Adacel as Compared to Td Vaccine in Adolescents and Adults 11 through 64 Years of Age (Td506) Anti-Diphtheria toxin (IU/mL)
Pre-vaccinationAnti-Diphtheria toxin (IU/mL)
Pre-vaccinationAnti-Diphtheria toxin (IU/mL)
1 Month Post-vaccinationAnti-Diphtheria toxin (IU/mL)
1 Month Post-vaccinationAnti-Diphtheria toxin (IU/mL)
1 Month Post-vaccinationAge Group
(years)Vaccine N* % ≥0.10
(95% CI)% ≥1.0
(95% CI)% ≥0.10
(95% CI)% ≥1.0
(95% CI)% Booster†
(95% CI)- *
- N = number of participants in the per-protocol population with available data.
- †
- Booster response is defined as: A 4-fold rise in antibody concentration, if the pre-vaccination concentration was equal to or below the cut-off value and a 2-fold rise in antibody concentration if the pre-vaccination concentration was above the cut-off value. The cut-off value for diphtheria was 2.56 IU/mL.
- ‡
- Seroprotection rates at ≥0.10 IU/mL and booster response rates to Adacel were non-inferior to Td vaccine (upper limit of the 95% CI on the difference for Td vaccine minus Adacel <10%).
- §
- Seroprotection rates at ≥1.0 IU/mL were not prospectively defined as a primary endpoint.
- ¶
- Tetanus and Diphtheria Toxoids Adsorbed manufactured by Sanofi Pasteur Inc., Swiftwater, PA.
11-17 Adacel 527 72.5
(68.5, 76.3)15.7
(12.7, 19.1)99.8‡
(98.9, 100.0)98.7§
(97.3, 99.5)95.1‡
(92.9, 96.8)11-17 Td¶ 515-516 70.7
(66.5, 74.6)17.3
(14.1, 20.8)99.8
(98.9, 100.0)98.4
(97.0, 99.3)95.0
(92.7, 96.7)18-64 Adacel 739-741 62.6
(59.0, 66.1)14.3
(11.9, 17.0)94.1‡
(92.1, 95.7)78.0§
(74.8, 80.9)87.4‡
(84.8, 89.7)18-64 Td¶ 506-507 63.3
(59.0, 67.5)16.0
(12.9, 19.5)95.1
(92.8, 96.8)79.9
(76.1, 83.3)83.4
(79.9, 86.5)Table 6: Ratio of Pertussis Antibody Geometric Mean Concentrations (GMCs)* Observed One Month Following a First Vaccination with Adacel in Adolescents and Adults 11 through 64 Years of Age Compared with Those Observed in Infants One Month Following Vaccination at 2,4 and 6 Months of Age in the Efficacy Trial with DAPTACEL (Sweden I Efficacy Study) Adolescents 11-17 Years of Age
Adacel†/DAPTACEL‡
GMC Ratio
(95% CIs)Adults 18-64 Years of Age
Adacel§/DAPTACEL‡
GMC Ratio
(95% CIs)- *
- Antibody GMCs, measured in arbitrary ELISA units were calculated separately for infants, adolescents and adults.
- †
- N = 524 to 526, number of adolescents in the per-protocol population with available data for Adacel.
- ‡
- N = 80, number of infants who received DAPTACEL with available data post dose 3 (Sweden Efficacy I).
- §
- N = 741, number of adults in the per-protocol population with available data for Adacel.
- ¶
- GMC following Adacel was non-inferior to GMC following DAPTACEL (lower limit of 95% CI on the ratio of GMC for Adacel divided by DAPTACEL >0.67).
Anti-PT 3.6
(2.8, 4.5)¶2.1
(1.6, 2.7)¶Anti-FHA 5.4
(4.5, 6.5)¶4.8
(3.9, 5.9)¶Anti-PRN 3.2
(2.5, 4.1)¶3.2
(2.3, 4.4)¶Anti-FIM 5.3
(3.9, 7.1)¶2.5
(1.8, 3.5)¶Table 7: Booster Response Rates to the Pertussis Antigens Observed One Month Following a First Vaccination with Adacel in Adolescents and Adults 11 through 64 Years of Age Adolescents 11-17
Years of Age
N*Adolescents 11-17
Years of Age
%
(95% CI)Adults 18-64
Years of Age
N*Adults 18-64
Years of Age
%
(95% CI)Predefined
Acceptable Rates†
%‡- *
- N = number of participants in the per-protocol population with available data.
- †
- The acceptable response rate for each antigen was defined as the lower limit of the 95% CI for the rate being no more than 10% lower than the response rate observed in previous clinical trials.
- ‡
- A booster response for each antigen was defined as a 4-fold rise in antibody concentration if the pre-vaccination concentration was equal to or below the cut-off value and a 2-fold rise in antibody concentration if the pre-vaccination concentration was above the cut-off value. The cut-off values for pertussis antigens were established based on antibody data from both adolescents and adults in previous clinical trials. The cut-off values were 85 EU/mL for PT, 170 EU/mL for FHA, 115 EU/mL for PRN and 285 EU/mL for FIM.
Anti-PT 524 92.0
(89.3, 94.2)739 84.4
(81.6, 87.0)81.2 Anti-FHA 526 85.6
(82.3, 88.4)739 82.7
(79.8, 85.3)77.6 Anti-PRN 525 94.5
(92.2, 96.3)739 93.8
(91.8, 95.4)86.4 Anti-FIM 526 94.9
(92.6, 96.6)739 85.9
(83.2, 88.4)82.4 Study NCT01311557 assessed the comparative immunogenicity of a first vaccination with Adacel administered to adolescents (10 to <11 years of age and 11 to <12 years of age) [See ADVERSE REACTIONS (6.1).] In this study non-inferiority was demonstrated for booster responses to tetanus and diphtheria toxoids, GMCs to the pertussis antigens (PT, FHA, PRN and FIM) and booster responses to the pertussis antigens PT, FHA and PRN. For FIM, non-inferiority was not demonstrated as the lower bound of the 95% CI of the difference in booster response rates (-5.96%) did not meet the predefined criterion (>-5% when the booster response in the older age group was >95%).
14.2 Immunological Evaluation in Adults, 18 through 64 Years of Age Following a Second Vaccination with Adacel
In study NCT01439165 [See ADVERSE REACTIONS (6.1).], subjects 18 to 64 years of age who had received a dose of Adacel 8-12 years previously, were randomized to receive a second dose of Adacel or Td vaccine (Tetanus and Diphtheria Toxoids Adsorbed manufactured by Sanofi Pasteur, Limited). Blood samples for immunogenicity analyses were obtained from participants pre-vaccination and approximately 28 days post-vaccination. The per-protocol analysis set was used for all immunogenicity analyses, and included 948 participants in the Adacel group and 317 participants in the Td control vaccine group. Of the study participants, 35% were male. Of subjects who reported a racial/ethnic demographic, 95% were Caucasian, 2% Black, 0.5% American Indian or Alaska native, 1% Asian and 1.5% were of mixed or other origin.
A tetanus antitoxoid level of ≥0.1 IU/mL, measured by the ELISA used in this study was considered protective. An anti-diphtheria anti-toxin level of ≥0.1 IU/mL was considered protective. Pre-vaccination and post-vaccination seroprotection rates and booster response rates are presented in Table 8.
Table 8: Pre-vaccination and Post-vaccination Seroprotection Rates and Booster Response Rates to Tetanus Toxoid and Diphtheria Toxoid Following a Second Vaccination with Adacel Compared to Td Vaccine in Persons 18 through 64 Years of Age, Per Protocol Analysis Set Vaccine N* Pre-vaccination ≥0.1 IU/mL
(95% CI)Pre-vaccination ≥1.0 IU/mL
(95% CI)1 month post-vaccination ≥0.1 IU/mL
(95% CI)†1 month post-vaccination ≥1.0 IU/mL
(95% CI)‡1 month post-vaccination %Booster§
(95% CI)- *
- N = number of participants in the per-protocol population with available data.
- †
- Seroprotection rates at ≥0.10 IU/mL for Adacel were non-inferior to Td for diphtheria toxin and tetanus toxoid (upper limit of the 95% CI on the difference for Td vaccine minus Adacel <10%).
- ‡
- Seroprotection rates at ≥1.0 IU/mL were not prospectively defined as a primary or secondary endpoint.
- §
- Booster response is defined as a minimum rise in antibody concentration from pre to post-vaccination. The minimum rise is at least 2 times if the pre-vaccination concentration is above the cutoff value, or at least 4 times if it is at or below the cutoff value. The cutoff values for to tetanus and diphtheria are 2.7 IU/mL and 2.56 IU/mL, respectively.
- ¶
- n/M: defines the number n of participants with booster response / the number M of subjects with available data to evaluate booster response. There were (n/M) 703/944, 257/315, 786/945 and 265/315 for Adacel/Tetanus, Td Adsorbed/Tetanus, Adacel/Diphtheria, and Td Adsorbed/Diphtheria, respectively.
- #
- Booster response rates for tetanus toxoid in Adacel did not meet the pre-specified non-inferiority criteria.
- Þ
- Tetanus and Diphtheria Toxoids Adsorbed manufactured by Sanofi Pasteur Limited, Toronto, Ontario, Canada.
Anti- Tetanus Toxoid (ELISA - IU/mL) Adacel 944-948 97.2
(96.0; 98.2)62.3
(59.1; 65.4)100.0
(99.6; 100.0)99.9
(99.4; 100.0)74.5¶ #
(71.6; 77.2)Anti- Tetanus Toxoid (ELISA - IU/mL) TdÞ Adsorbed 315-317 96.5
(93.8; 98.2)63.8
(58.2; 69.1)100.0
(98.8; 100.0)100.0
(98.8; 100.0)81.6¶ #
(76.9; 85.7)Anti- Diphtheria Toxin (ELISA - IU/mL) Adacel 945-948 84.7
(82.2; 86.9)29.1
(26.2; 32.1)99.8
(99.2; 100.0)94.9
(93.3; 96.2)83.2¶
(80.6; 85.5)Anti- Diphtheria Toxin (ELISA - IU/mL) TdÞ Adsorbed 315-317 83.8
(79.3; 87.7)29.8
(24.8; 35.2)99.4
(97.7; 99.9)94.0
(90.8; 96.4)84.1¶
(79.6; 88.0)For all pertussis antigens (PT, FHA, PRN and FIM), post-vaccination anti-pertussis GMCs in the Adacel group were non-inferior to GMCs induced by 3 or 4 doses of DAPTACEL in historical studies as are presented in Table 9.
Table 9: Ratio of Pertussis Antibody Geometric Mean Concentrations (GMCs) Observed One Month Following a Second Vaccination with Adacel in Adults Compared with Those Observed in Infants One Month following Vaccination with 3 or 4 Doses of DAPTACEL (Per-Protocol Analysis Set) Antigen N Adacel
GMC
(EU/mL)Adacel
(95% CI)N DAPTACEL*
GMC
(EU/mL)DAPTACEL*
(95% CI)Adacel/DAPTACEL*
GMC Ratio(95% CI)† - *
- DAPTACEL: Historical controls who received DAPTACEL in Sanofi Pasteur studies. PT antibody GMC were compared to GMC following 4 doses of DAPTACEL in NCT00255047. FHA, PRN and FIM antibody GMCs were compared to GMCs following 3 doses of Daptacel in the Sweden I Efficacy trial.
- †
- For each pertussis antigen, non-inferiority was demonstrated if the lower limit of the 2-sided 95% CI of the GMC ratio (Adacel divided by the historical control) was > 0.66.
PT 935 102 (94.9; 110) 366 98.1 (90.9; 106) 1.04 (0.92; 1.18) FHA 948 209 (200; 217) 80 39.9 (34.6; 46.1) 5.22 (4.51; 6.05) PRN 948 318 (302; 334) 80 108 (91.4; 128) 2.94 (2.46; 3.51) FIM 948 745 (711; 781) 80 341 (270; 431) 2.18 (1.84; 2.60) Booster response rates for PT and FHA were non-inferior in Adacel participants compared to pre-specified criteria for booster response rates, but non-inferiority was not achieved for PRN and FIM booster response rates (See Table 10).
Table 10: Comparison of Booster Response* Rates for Pertussis Antigens Following a Second Vaccination with Adacel (Per-Protocol Analysis Set) Adacel
(N=948)Adacel
(N=948)Pre-specified criteria for Booster Response Rates† Adacel minus Pre-specified Booster Response Rates† Adacel minus Pre-specified Booster Response Rates† Antigen n/M % (95% CI) % Difference (%) (95% CI)‡ N = number of subjects analyzed according to Per-Protocol Analysis Set
M = number of subjects with available data for the considered endpoint
n = number of subjects fulfilling the item listed in the first column- *
- Booster response is defined as a minimum rise in antibody concentration from pre to post-vaccination. The minimum rise is at least 2-fold if the pre-vaccination concentration is above the cutoff value, or at least 4-fold if it is at or below the cutoff value. The cutoff values for Study NCT01439165 for the pertussis antigens are: 93 EU/mL for PT, 170 EU/mL for FHA, 115 EU/mL for PRN, and 285 EU/mL for FIM.
- †
- Pre-specified criteria for booster response rates were derived from participants 21 to <65 years of age who received Adacel in Study Td506.
- ‡
- Non-inferiority in booster response rate for each pertussis antigen was demonstrated if the lower limit of the 2-sided 95% CI of the difference of booster response rates between participants receiving Adacel in Study NCT01439165 and expected booster response rates based on Study Td506 was >-10%.
PT 693/894 77.5 (74.6; 80.2) 61.4 16.12 (13.27; 18.73) FHA 651/945 68.9 (65.8; 71.8) 73.1 -4.21 (-7.23; -1.34) PRN 617/945 65.3 (62.2; 68.3) 83.9 -18.61 (-21.7; -15.6) FIM 537/945 56.8 (53.6; 60.0) 75.9 -19.07 (-22.3; -16.0) 14.3 Study in Pregnant Women
The effectiveness of Adacel immunization during the third trimester of pregnancy to prevent pertussis among infants younger than 2 months of age was assessed based on a re-analysis of the Adacel-relevant data (Study NCT05040802) from an observational study of Tdap vaccine effectiveness in the United States (9). This matched case-control study included pertussis cases reported from 2011 through 2014 in infants born at ≥ 37 weeks gestation and ≥ 2 days old. A case of pertussis was defined as the onset of cough illness and at least one of the following: laboratory-confirmation (culture or PCR) of pertussis, epidemiological linkage to a laboratory-confirmed case, or clinically-compatible illness (cough ≥2 weeks with paroxysms, inspiratory whoop or post-tussive vomiting) in an infant <2 months old. NCT05040802 included a total of 596 infants (179 cases of pertussis and 417 controls without pertussis), the mean age was 4.2 (SD, 2.5) weeks, and 45.7% were non-Hispanic. For the vaccine effectiveness re-analysis, a conditional logistic regression model controlling for infant's age and maternal education, was fit to data from 101 cases (including 5 infants whose mothers received Adacel during the third trimester and ≥14 days before delivery) and 171 controls (including 27 infants whose mothers received Adacel during the third trimester and ≥14 days before delivery) matched by birth hospital. This resulted in a vaccine effectiveness estimate of 88.0% (95% CI: 43.8, 97.4) for vaccination during the third trimester and ≥14 days before delivery.
14.4 Immune Responses to Pertussis Vaccination in Infants Born to Mothers Who Received Adacel During Pregnancy
Published studies have reported diminished immune responses to pertussis antigens in DTaP-containing vaccines administered to infants whose mothers received Adacel during the third trimester of pregnancy compared with infants whose mothers did not receive Adacel during the third trimester of pregnancy (10, 11). Whether the diminished immune responses observed in vaccinated infants whose mothers received Adacel during pregnancy result in diminished effectiveness of pertussis vaccination in infants is unknown.
14.5 Concomitant Hepatitis B Vaccine Administration
The concomitant use of Adacel (first vaccination) and hepatitis B (Hep B) vaccine (Recombivax HB®, 10 mcg per dose using a two-dose regimen, manufactured by Merck and Co., Inc.) was evaluated in a multi-center, open-labeled, randomized, controlled study that enrolled 410 adolescents, 11 through 14 years of age inclusive. One group received Adacel and Hep B vaccines concurrently (N = 206). The other group (N = 204) received Adacel at the first visit, then 4-6 weeks later received Hep B vaccine. The second dose of Hep B vaccine was given 4-6 weeks after the first dose. Serum samples were obtained prior to and 4-6 weeks after Adacel administration, as well as 4-6 weeks after the 2nd dose of Hep B for all participants. No interference was observed in the immune responses to any of the vaccine antigens when Adacel and Hep B vaccines were given concurrently or separately. [See ADVERSE REACTIONS (6.1).]
14.6 Concomitant Influenza Vaccine Administration
The concomitant use of Adacel (first vaccination) and trivalent inactivated influenza vaccine (TIV, Fluzone®, manufactured by Sanofi Pasteur Inc., Swiftwater, PA) was evaluated in a multi-center, open-labeled, randomized, controlled study conducted in 720 adults, 19-64 years of age inclusive. In one group, participants received Adacel and TIV vaccines concurrently (N = 359). The other group received TIV at the first visit, then 4-6 weeks later received Adacel (N = 361). Sera were obtained prior to and 4-6 weeks after Adacel, as well as 4-6 weeks after the TIV. The immune responses were comparable for concurrent and separate administration of Adacel and TIV vaccines for diphtheria (percent of participants with seroprotective concentration ≥0.10 IU/mL and booster responses), tetanus (percent of participants with seroprotective concentration ≥0.10 IU/mL), pertussis antigens (booster responses and GMCs except lower PRN GMC in the concomitant group, lower bound of the 90% CI was 0.61 and the prespecified criterion was ≥0.67) and influenza antigens (percent of participants with hemagglutination-inhibition [HI] antibody titer ≥1:40 IU/mL and ≥4-fold rise in HI titer). Although tetanus booster response rates were significantly lower in the group receiving the vaccines concurrently versus separately, greater than 98% of participants in both groups achieved seroprotective levels of ≥0.1 IU/mL. [See ADVERSE REACTIONS (6.1).]
-
15 REFERENCES
- 1
- Stratton KR, et al, editors. Adverse events associated with childhood vaccines; evidence bearing on causality. Washington: National Academy Press; 1994. p. 67-117.
- 2
- Stainer DW, et al. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol 1970;63:211-20.
- 3
- Mueller JH, et al. Variable factors influencing the production of tetanus toxin. J Bacteriol 1954;67(3):271-7.
- 4
- Stainer DW. Production of diphtheria toxin. In: Manclark CR, editor. Proceedings of an informal consultation on the World Health Organization requirements for diphtheria, tetanus, pertussis and combined vaccines. United States Public Health Service, Bethesda, MD. DHHS 91-1174. 1991. p. 7-11.
- 5
- FDA. Department of Health and Human Services (DHHS). Biological products bacterial vaccines and toxoids; implementation of efficacy review; proposed rule. Fed Reg 1985;50(240):51002-117.
- 6
- Wassilak SGF, et al. Tetanus toxoid. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia, PA: WB Saunders Company; 2008. p. 805-39.
- 7
- Vitek CR and Wharton M. Diphtheria toxoid. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia, PA: W.B. Saunders Company; 2008. p. 139-56.
- 8
- Gustafsson L, et al. A controlled trial of a two-component acellular, a five-component acellular and a whole-cell pertussis vaccine. N Engl J Med 1996;334(6):349-55.
- 9
- Skoff TH, Kenyon C, Cocoros N, Liko J, Miller L, Kudish K, et al. Sources of infant pertussis infection in the United States. Pediatrics. 2015;136(4):635-41.
- 10
- Halperin, SA et al. A randomized controlled trial of the safety and immunogenicity of tetanus, diphtheria, and acellular pertussis vaccine immunization during pregnancy and subsequent infant immune response. Clin Infect Dis. 2018;67(7):1063-71.
- 11
- Munoz, FM et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA. 2014;311(17):1760-9.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Syringe, without needle, single-dose – NDC 49281-400-89 (not made with natural rubber latex); in package of 5 syringes, NDC 49281-400-20.
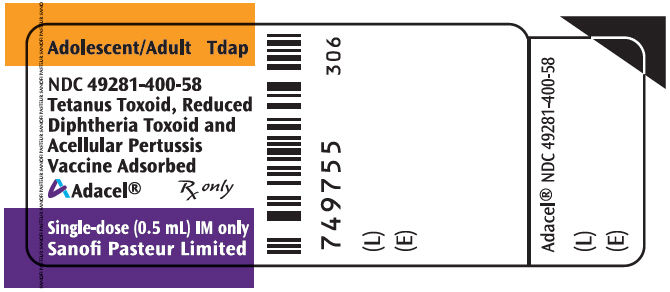
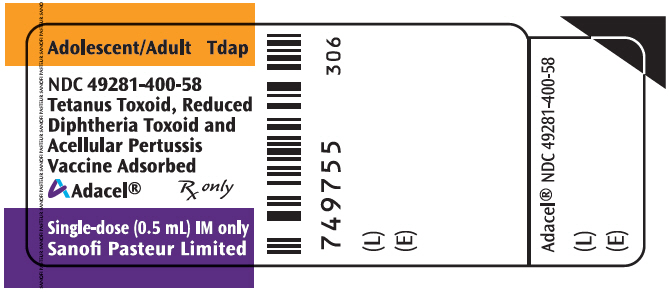
Vial, single-dose – NDC 49281-400-58; in package of 5 vials; NDC 49281-400-05. The vial stopper is not made with natural rubber latex. Discard unused portion in vial.
Vial, single-dose – NDC 49281-400-58; in package of 10 vials; NDC 49281-400-10. The vial stopper is not made with natural rubber latex. Discard unused portion in vial.
Not all pack sizes may be marketed.
-
17 PATIENT COUNSELING INFORMATION
Before administration of Adacel, healthcare providers should inform the patient, parent or guardian of the benefits and risks of the vaccine and the importance of receiving recommended booster dose unless a contraindication to further immunization exists.
The healthcare provider should inform the patient, parent or guardian about the potential for adverse reactions that have been temporally associated with Adacel or other vaccines containing similar components. The healthcare provider should provide the Vaccine Information Statements (VISs) that are required by the National Childhood Vaccine Injury Act of 1986 to be given with each immunization. The patient, parent or guardian should be instructed to report any serious adverse reactions to their healthcare provider.
- SPL UNCLASSIFIED SECTION
-
Patient Information Sheet Adacel® Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed
Please read this information before vaccination with Adacel vaccine. This summary is not intended to take the place of talking with your healthcare provider. If you have questions or would like more information, please talk with your healthcare provider.
What is Adacel vaccine?
Adacel vaccine is a vaccine that helps protect against tetanus, diphtheria, and pertussis diseases in people who are 10 through 64 years of age. It cannot cause tetanus, diphtheria, or pertussis. Adacel vaccine may not protect all people getting the vaccine.
Adacel given during the third trimester of pregnancy helps the pregnant woman create antibodies against the bacteria that cause whooping cough (pertussis) that are passed to her baby before birth. These antibodies help protect the baby against whooping cough during the first few months of life.
Tetanus, also called "lockjaw", can cause severe muscle spasms making it difficult for a person to open their mouth or swallow. You can get tetanus through a cut or wound.
Diphtheria can cause throat, lung and skin infections leading to severe complications that affect the lungs, heart and nervous system.
Pertussis, also called "whooping cough," causes coughing fits that can affect breathing. Diphtheria and pertussis are spread from person to person.
Who should not get Adacel vaccine?
You should not get Adacel vaccine if you:
- had a severe allergic reaction to a previous tetanus vaccine, diphtheria vaccine, pertussis vaccine, or any component of Adacel vaccine.
- were told you have an "encephalopathy", which is a kind of brain disease or malfunction, after receiving a previous dose of a pertussis vaccine.
- are younger than 10 years old or older than 64 years of age.
What should I tell my healthcare provider before I or my child gets Adacel vaccine?
Tell your healthcare provider if you or your child:
- had severe injection site pain or swelling after a prior tetanus, diphtheria, or pertussis vaccination.
- had Guillain-Barré syndrome, a nerve disease causing severe muscle weakness, after getting a vaccine.
- have a brain disorder or brain disease that is not stable.
- are pregnant or nursing.
- had a tetanus, diphtheria, or pertussis vaccine within the last 5 years.
Fainting can occur around the time of vaccination with Adacel or other vaccines. Tell your healthcare provider if you or your child has fainted in connection with any previous vaccination.
How is Adacel vaccine given?
Adacel is a single shot that is given into the muscle of the upper arm.
What are the possible side effects of Adacel vaccine?
The most common side effects of Adacel vaccine are:
- pain, redness and swelling where you got the shot
- headache
- body ache
- tiredness
- fever
These are not all the possible side effects of Adacel vaccine. You may ask your healthcare provider for a list of side effects that is available to healthcare professionals.
If you or your child experience side effects that concern you, call your healthcare provider for medical advice. You may report side effects to VAERS at 1-800-822-7967 or http://vaers.hhs.gov.
What ingredients are in Adacel vaccine?
Adacel vaccine contains noninfectious tetanus, diphtheria, and pertussis proteins, aluminum phosphate, 2-phenoxyethanol, and residual amounts of formaldehyde and glutaraldehyde.
Adacel vaccine does not contain preservatives.
Manufactured by:
Sanofi Pasteur Limited
Toronto Ontario Canada
Distributed by:
Sanofi Pasteur Inc.
Swiftwater PA 18370 USAR13-1220 USA
- PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Package
- PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Label
- PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Package
-
INGREDIENTS AND APPEARANCE
ADACEL TDAP
clostridium tetani toxoid antigen (formaldehyde inactivated), corynebacterium diphtheriae toxoid antigen (formaldehyde inactivated), bordetella pertussis toxoid antigen (glutaraldehyde inactivated), bordetella pertussis filamentous hemagglutinin antigen (formaldehyde inactivated), bordetella pertussis pertactin antigen, and bordetella pertussis fimbriae 2/3 antigen injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:49281-400 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: K3W1N8YP13) (CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:K3W1N8YP13) CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) 5 [Lf] in 0.5 mL CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: IRH51QN26H) (CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:IRH51QN26H) CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) 2 [Lf] in 0.5 mL BORDETELLA PERTUSSIS TOXOID ANTIGEN (GLUTARALDEHYDE INACTIVATED) (UNII: F4TN0IPY37) (BORDETELLA PERTUSSIS TOXOID ANTIGEN (GLUTARALDEHYDE INACTIVATED) - UNII:F4TN0IPY37) BORDETELLA PERTUSSIS TOXOID ANTIGEN (GLUTARALDEHYDE INACTIVATED) 2.5 ug in 0.5 mL BORDETELLA PERTUSSIS FILAMENTOUS HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 8C367IY4EY) (BORDETELLA PERTUSSIS FILAMENTOUS HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:8C367IY4EY) BORDETELLA PERTUSSIS FILAMENTOUS HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 5 ug in 0.5 mL BORDETELLA PERTUSSIS PERTACTIN ANTIGEN (UNII: 63GD90PP8X) (BORDETELLA PERTUSSIS PERTACTIN ANTIGEN - UNII:63GD90PP8X) BORDETELLA PERTUSSIS PERTACTIN ANTIGEN 3 ug in 0.5 mL BORDETELLA PERTUSSIS FIMBRIAE 2/3 ANTIGEN (UNII: 1O0600285A) (BORDETELLA PERTUSSIS FIMBRIAE 2/3 ANTIGEN - UNII:1O0600285A) BORDETELLA PERTUSSIS FIMBRIAE 2/3 ANTIGEN 5 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength ALUMINUM PHOSPHATE (UNII: F92V3S521O) 1.5 mg in 0.5 mL FORMALDEHYDE (UNII: 1HG84L3525) 5 ug in 0.5 mL GLUTARAL (UNII: T3C89M417N) 50 ng in 0.5 mL PHENOXYETHANOL (UNII: HIE492ZZ3T) 3.3 mg in 0.5 mL Product Characteristics Color WHITE (WHITE (CLOUDY)) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49281-400-10 10 in 1 PACKAGE 1 NDC:49281-400-58 0.5 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC:49281-400-05 5 in 1 PACKAGE 2 NDC:49281-400-58 0.5 mL in 1 VIAL; Type 0: Not a Combination Product 3 NDC:49281-400-20 5 in 1 PACKAGE 3 NDC:49281-400-89 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125111 06/10/2005 Labeler - Sanofi Pasteur Inc. (086723285) Establishment Name Address ID/FEI Business Operations Sanofi Pasteur Limited 208206623 MANUFACTURE(49281-400)