Label: HOLIDAY DUST SET- alcohol kit
- NDC Code(s): 72866-030-01, 72866-031-01, 72866-032-01, 72866-033-01
- Packager: MERCI HANDY CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aqua (Water), Parfum (Fragrance), Aloe Barbadensis Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Zea Mays (Corn) Starch, Denatonium Benzoate, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, D&C Red No. 30, D&C Red No. 33.
- QUESTIONS OR COMMENTS?
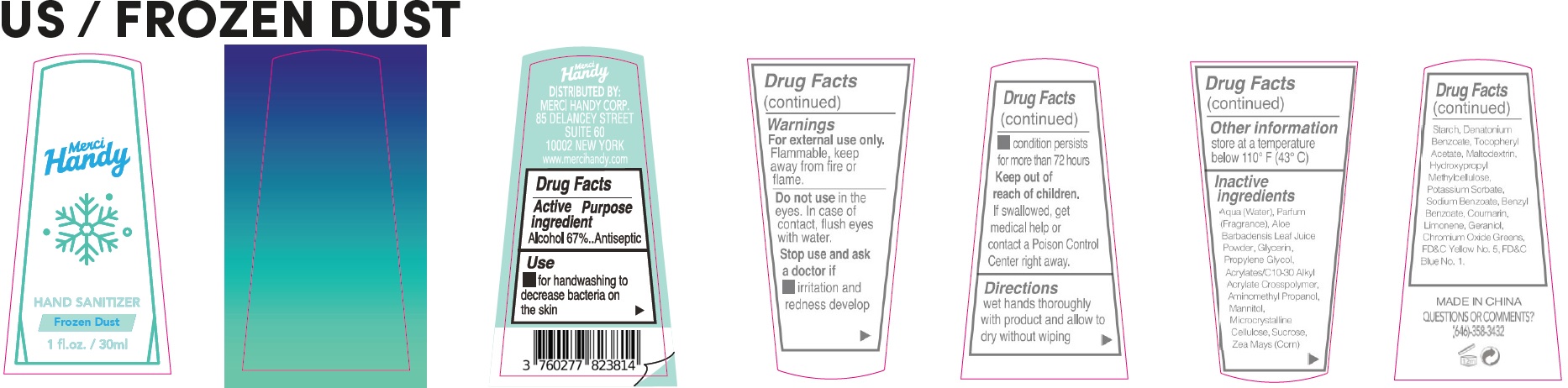
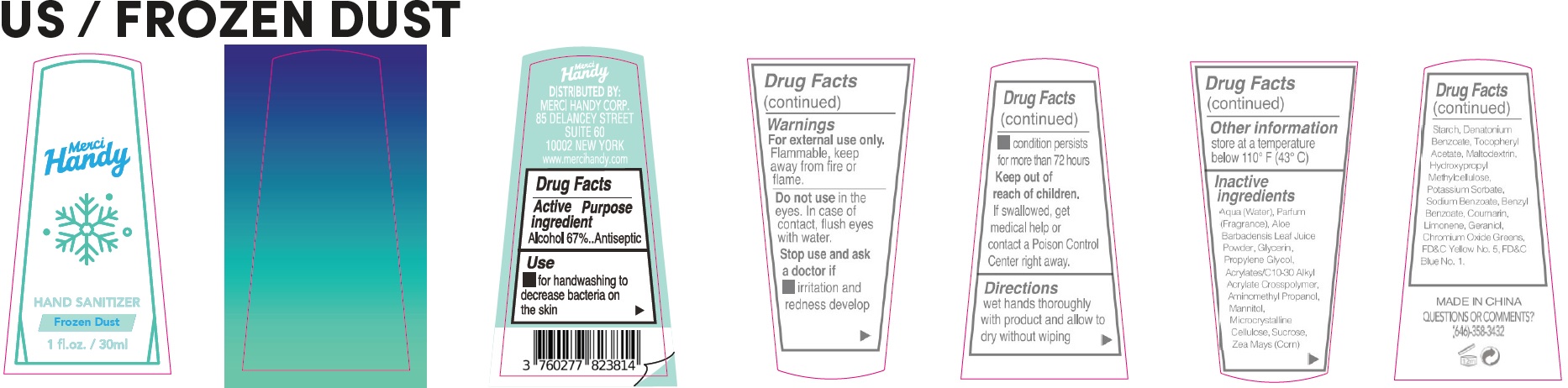
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aqua (Water), Parfum (Fragrance), Aloe Barbadensis Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Zea Mays (Corn) Starch, Denatonium Benzoate, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, Benzyl Benzoate, Coumarin, Limonene, Geraniol, Chromium Oxide Greens, FD&C Yellow No. 5, FD&C Blue No. 1.
- QUESTIONS OR COMMENTS?
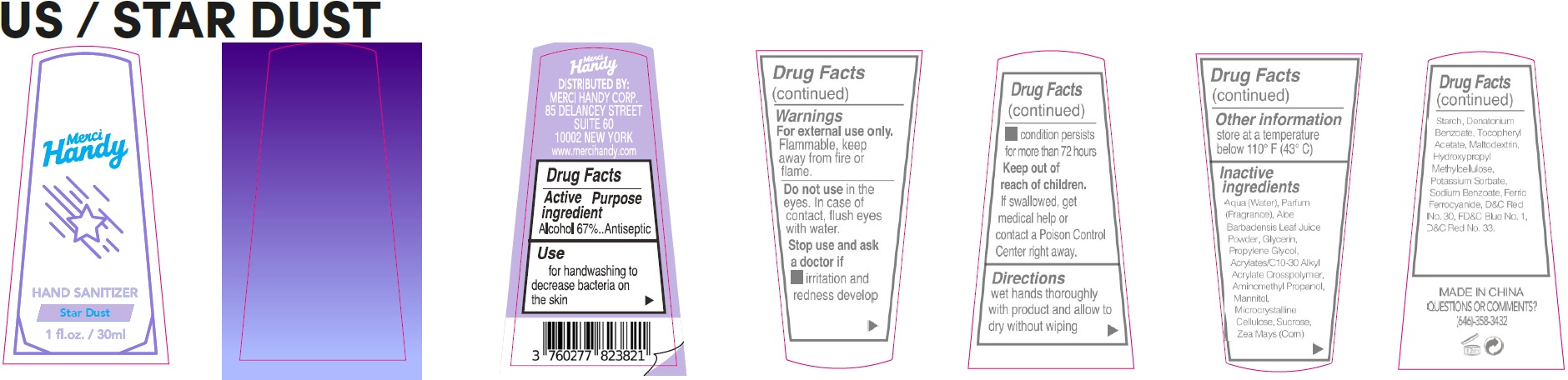
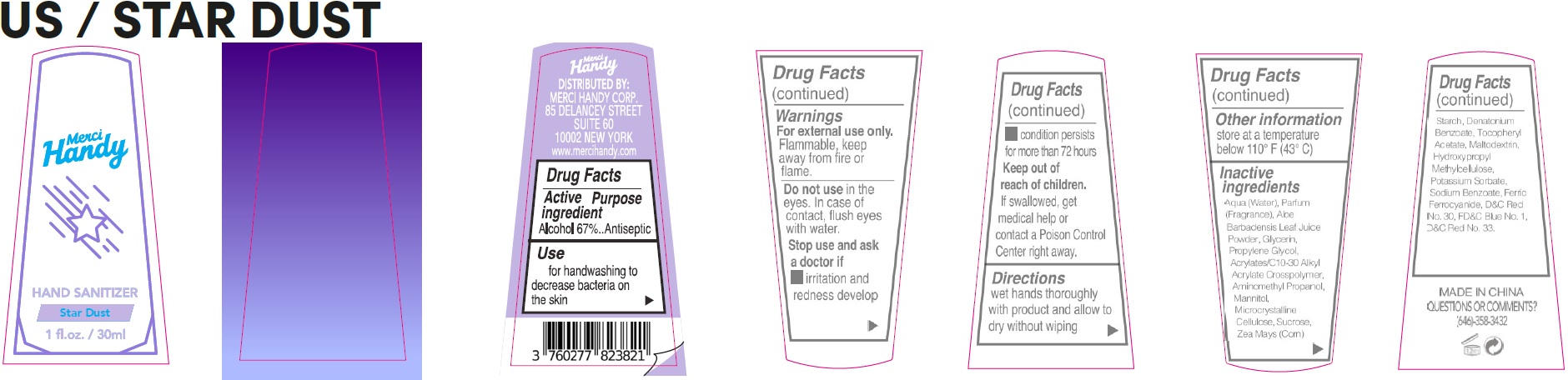
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aqua (Water), Parfum (Fragrance), Aloe Barbadensis Leaf Juice Powder, Glycerin, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Mannitol, Microcrystalline Cellulose, Sucrose, Zea Mays (Corn) Starch, Denatonium Benzoate, Tocopheryl Acetate, Maltodextrin, Hydroxypropyl Methylcellulose, Potassium Sorbate, Sodium Benzoate, Ferric Ferrocyanide, D&C Red No. 30, FD&C Blue No. 1, D&C Red No. 33.
- QUESTIONS OR COMMENTS?
- Package Labeling:72866-030-01
- Package Labeling:72866-031-01
- Package Labeling:72866-032-01
- Package Labeling:72866-033-01
-
INGREDIENTS AND APPEARANCE
HOLIDAY DUST SET
alcohol kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72866-030 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-030-01 1 in 1 KIT 09/20/2021 12/31/2026 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 30 mL Part 2 1 BOTTLE 30 mL Part 3 1 BOTTLE 30 mL Part 1 of 3 HAND SANITIZER DISCO DUST
alcohol gelProduct Information Item Code (Source) NDC:72866-031 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 67 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) CORN (UNII: 0N8672707O) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) D&C RED NO. 30 (UNII: 2S42T2808B) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-031-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/20/2021 Part 2 of 3 HAND SANITIZER FROZEN DUST
alcohol gelProduct Information Item Code (Source) NDC:72866-032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 67 mL in 100 mL Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) BENZYL BENZOATE (UNII: N863NB338G) COUMARIN (UNII: A4VZ22K1WT) LIMONENE, (+)- (UNII: GFD7C86Q1W) GERANIOL (UNII: L837108USY) CHROMIC OXIDE (UNII: X5Z09SU859) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) CORN (UNII: 0N8672707O) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-032-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/20/2021 Part 3 of 3 HAND SANITIZER STAR DUST
alcohol gelProduct Information Item Code (Source) NDC:72866-033 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 67 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) CORN (UNII: 0N8672707O) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MALTODEXTRIN (UNII: 7CVR7L4A2D) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) FERRIC FERROCYANIDE (UNII: TLE294X33A) D&C RED NO. 30 (UNII: 2S42T2808B) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72866-033-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/20/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/20/2021 12/31/2026 Labeler - MERCI HANDY CORPORATION (118006306)