Label: CARRY ON HAND SANITIZER- isopropyl alcohol liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 74332-000-00 - Packager: Encore Industrial Products, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 6, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
This is a hand sanitizer manufactured according to the Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (CoViD-19); Guidance for Industry.
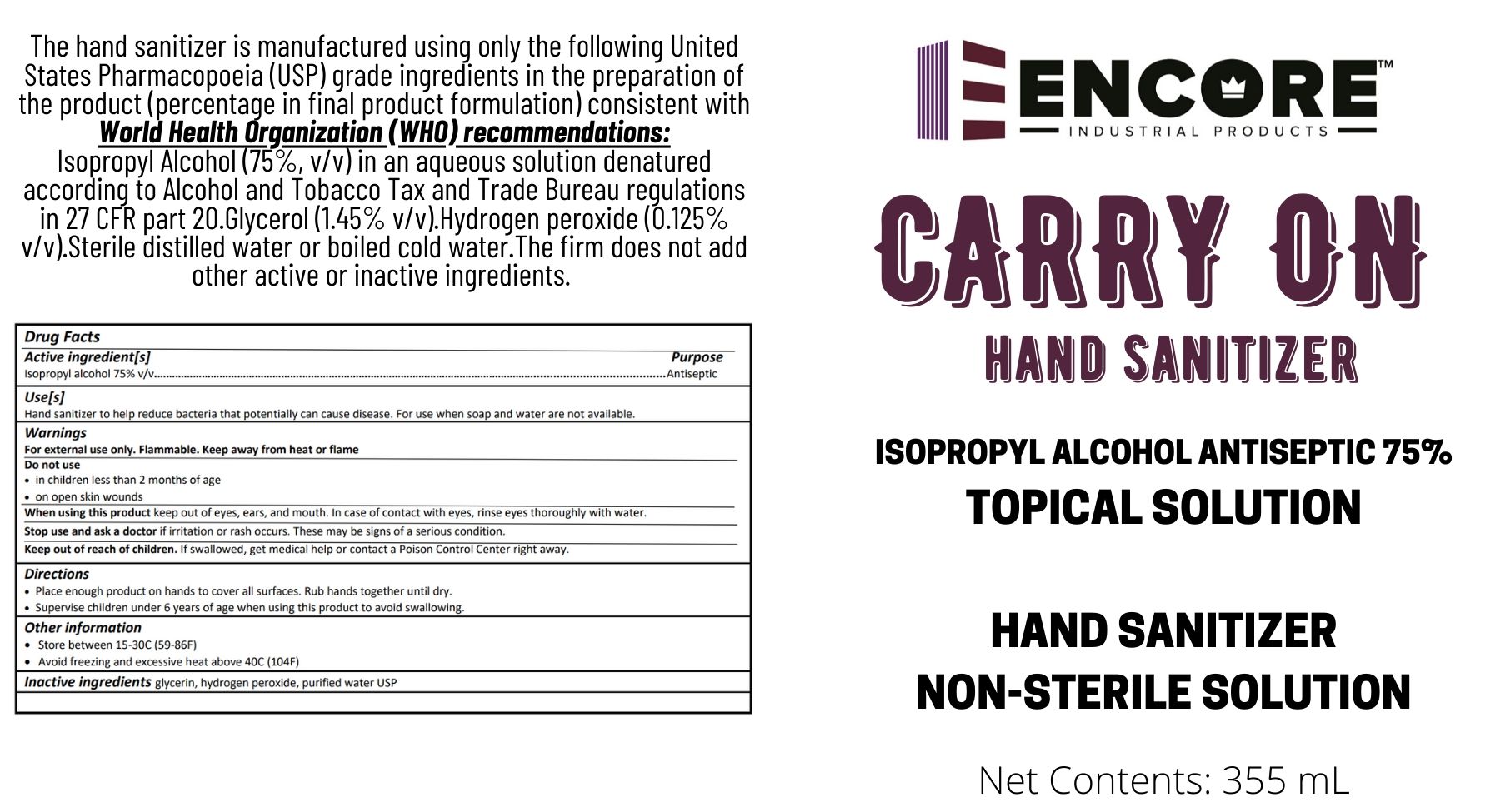
The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation) consistent with World Health Organization (WHO) recommendations:
- Isopropyl Alcohol (75%, v/v) in an aqueous solution denatured according to Alcohol and Tobacco Tax and Trade Bureau regulations in 27 CFR part 20.
- Glycerol (1.45% v/v).
- Hydrogen peroxide (0.125% v/v).
- Sterile distilled water or boiled cold water.
The firm does not add other active or inactive ingredients. Different or additional ingredients may impact the quality and potency of the product.

- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
-
WHEN USING
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CARRY ON HAND SANITIZER
isopropyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74332-000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 75 mL in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 1.45 mL in 100 mL HYDROGEN PEROXIDE (UNII: BBX060AN9V) 0.125 mL in 100 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74332-000-00 355 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/30/2020 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/30/2020 Labeler - Encore Industrial Products, LLC (066166082) Establishment Name Address ID/FEI Business Operations Encore Industrial Products, LLC 066166082 manufacture(74332-000)