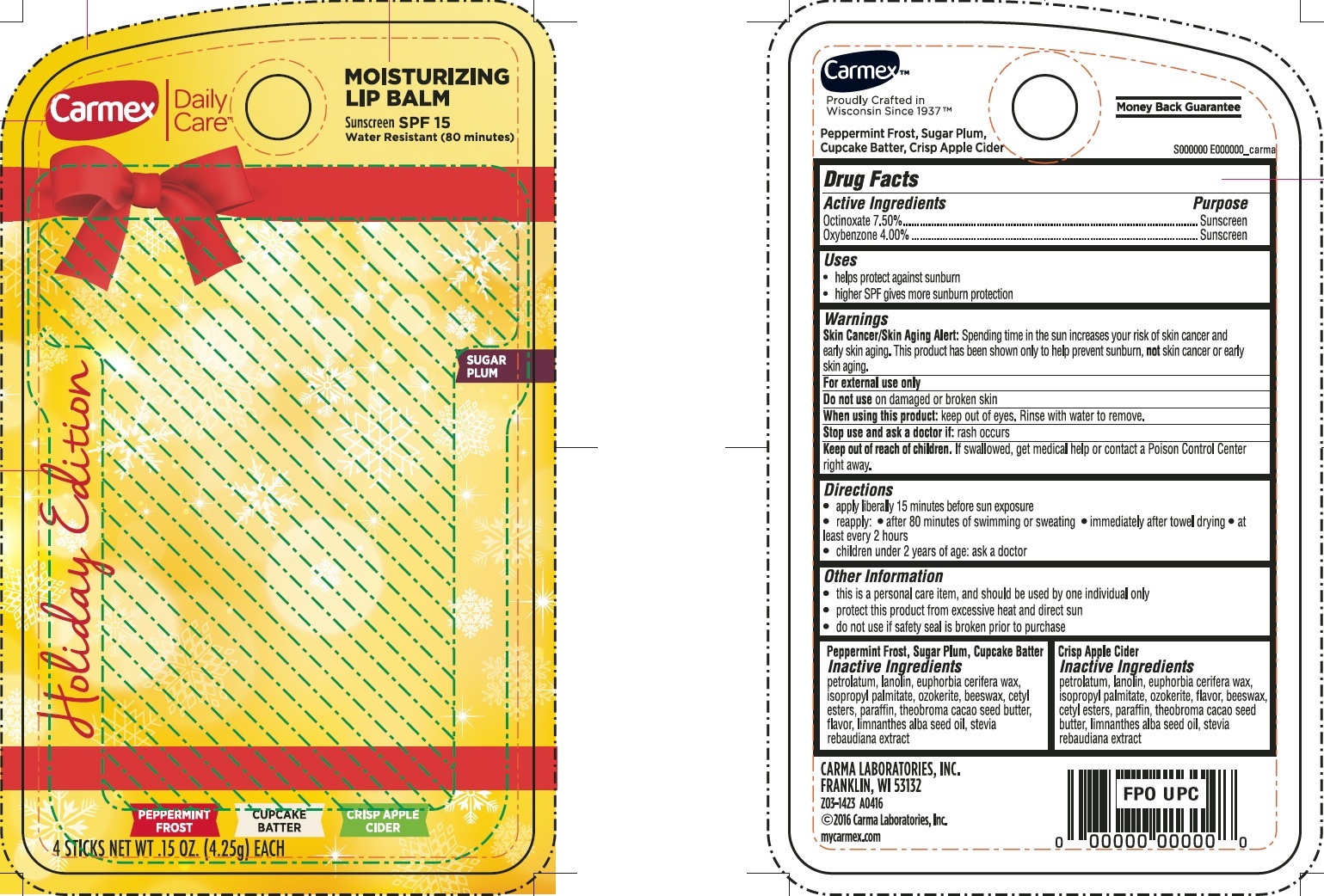
Label: CARMEX DAILY CARE MOISTURIZING LIP BALM SPF 15- octinoxate, oxybenzone kit
-
NDC Code(s):
10210-0017-1,
10210-0018-1,
10210-0019-1,
10210-0020-1, view more10210-0021-1
- Packager: Carma Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Package Labeling:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
CARMEX DAILY CARE MOISTURIZING LIP BALM SPF 15
octinoxate, oxybenzone kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10210-0017 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10210-0017-1 1 in 1 KIT 09/26/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 CYLINDER 4.25 g Part 2 1 CYLINDER 4.25 g Part 3 1 CYLINDER 4.25 g Part 4 1 CYLINDER 4.25 g Part 1 of 4 PEPERMINT FROST
octinoxate, oxybenzone salveProduct Information Item Code (Source) NDC:10210-0018 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) LANOLIN (UNII: 7EV65EAW6H) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) CERESIN (UNII: Q1LS2UJO3A) YELLOW WAX (UNII: 2ZA36H0S2V) CETYL ESTERS WAX (UNII: D072FFP9GU) PARAFFIN (UNII: I9O0E3H2ZE) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10210-0018-1 1 in 1 BLISTER PACK 1 4.25 g in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/26/2016 Part 2 of 4 CUPCAKE BATTER
octinoxate, oxybenzone salveProduct Information Item Code (Source) NDC:10210-0019 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) LANOLIN (UNII: 7EV65EAW6H) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) CERESIN (UNII: Q1LS2UJO3A) YELLOW WAX (UNII: 2ZA36H0S2V) CETYL ESTERS WAX (UNII: D072FFP9GU) PARAFFIN (UNII: I9O0E3H2ZE) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10210-0019-1 1 in 1 BLISTER PACK 1 4.25 g in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/26/2016 Part 3 of 4 CRISP APPLE CIDER
octinoxate, oxybenzone salveProduct Information Item Code (Source) NDC:10210-0020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) LANOLIN (UNII: 7EV65EAW6H) CANDELILLA WAX (UNII: WL0328HX19) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) CERESIN (UNII: Q1LS2UJO3A) YELLOW WAX (UNII: 2ZA36H0S2V) CETYL ESTERS WAX (UNII: D072FFP9GU) PARAFFIN (UNII: I9O0E3H2ZE) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10210-0020-1 1 in 1 BLISTER PACK 1 4.25 g in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/26/2016 Part 4 of 4 SUGAR PLUM
octinoxate, oxybenzone salveProduct Information Item Code (Source) NDC:10210-0021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) LANOLIN (UNII: 7EV65EAW6H) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) CERESIN (UNII: Q1LS2UJO3A) YELLOW WAX (UNII: 2ZA36H0S2V) CETYL ESTERS WAX (UNII: D072FFP9GU) PARAFFIN (UNII: I9O0E3H2ZE) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10210-0021-1 1 in 1 BLISTER PACK 1 4.25 g in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/26/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/26/2016 Labeler - Carma Laboratories, Inc. (006090153)