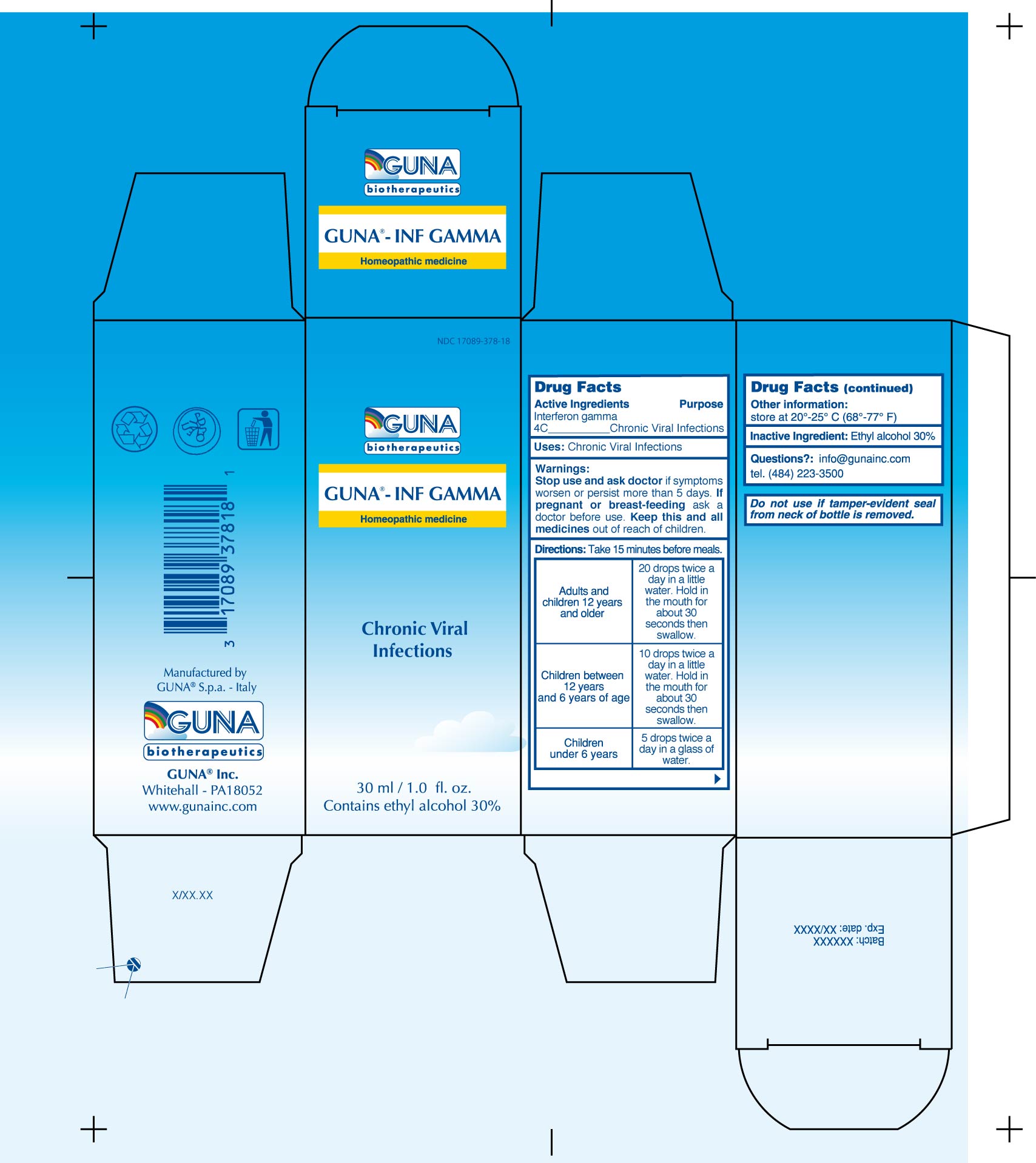
Label: GUNA-INF GAMMA- interferon gamma-1b solution/ drops
- NDC Code(s): 17089-378-18
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 15, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS/PURPOSE
- USES
- WARNINGS
- PREGNANCY
- WARNINGS
-
DIRECTIONS
Take 15 minutes before meals.
Adults and children 12 years and older 20 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children between 12 years and 6 years of age 10 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children under 6 years 5 drops twice a day in a glass of water.
- QUESTIONS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-INF GAMMA
interferon gamma-1b solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-378 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INTERFERON GAMMA-1B (UNII: 21K6M2I7AG) (INTERFERON GAMMA-1B - UNII:21K6M2I7AG) INTERFERON GAMMA-1B 4 [hp_C] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-378-18 1 in 1 BOX 06/17/2008 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/17/2008 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-378)