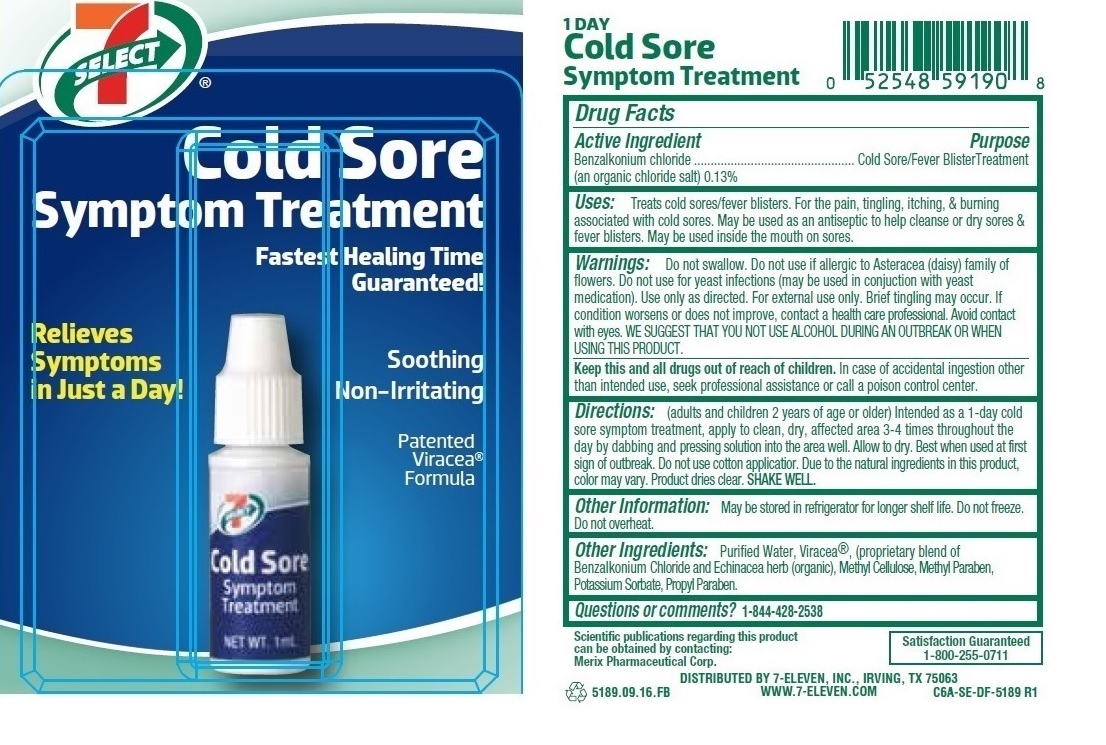
Label: 7 SELECT COLD SORE SYMPTOM TREATMENT- benzalkonium chloride solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 10202-591-90 - Packager: 7-eleven
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 2, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Purpose
- INDICATIONS & USAGE
-
WARNINGS
Warnings: Do not swallow. Do not use if allergic to Asteracea (daisy) family of flowers. Do not use for yeast infections (may be used in conjuction with yeast medication). Use only as directed. For external use only. Brief tingling may occur. If conditions worsens or does not improve, contact a health care professional. Avoid contact with eyes. WE SUGGEST THAT YOU NOT USE ALCOHOL DURING AN OUTBREAK OR WHEN USING THIS PRODUCT.
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions: (adults and children 2 years of age or older) Intended as a 1-day cold sore symptom treatment, apply to clean, dry, affected area 3-4 times throughout the day by dabbing and pressing solution into the area well. Allow to dry. Best when used at first sign of outbreak. Do not use cotton applicator. Due to the natural ingredients in this product, color may vary. Product dries clear. SHAKE WELL.
- INACTIVE INGREDIENT
- STORAGE AND HANDLING
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
7 SELECT COLD SORE SYMPTOM TREATMENT
benzalkonium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10202-591 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ECHINACEA PURPUREA FLOWERING TOP (UNII: 2EMS3QFX65) HYPROMELLOSE 2910 (4000 MPA.S) (UNII: RN3152OP35) METHYLPARABEN (UNII: A2I8C7HI9T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10202-591-90 1 in 1 BLISTER PACK 06/03/2016 1 1 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/03/2016 Labeler - 7-eleven (007347602) Establishment Name Address ID/FEI Business Operations Topical Pharmaceuticals, Inc 831530683 manufacture(10202-591)