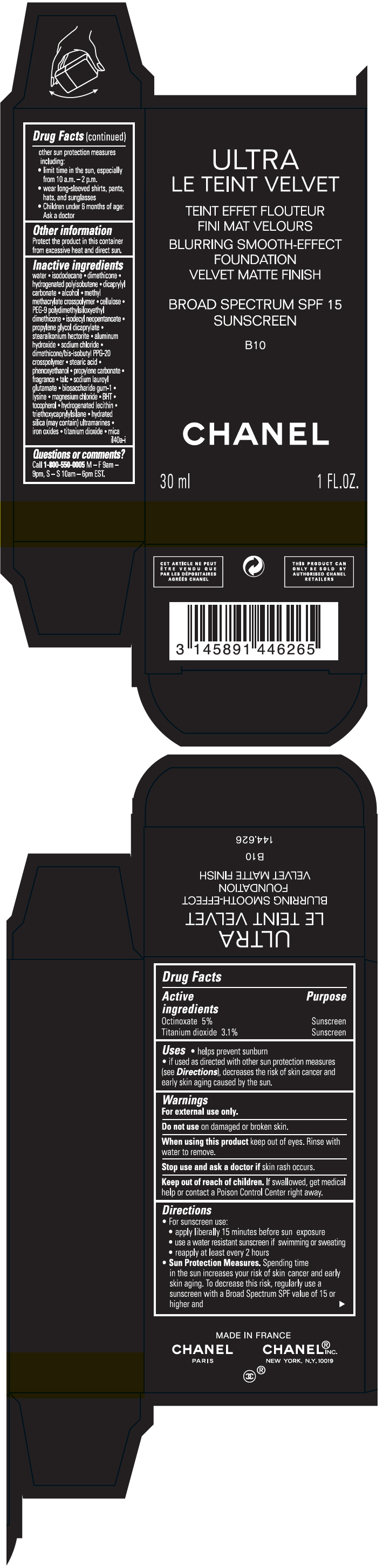
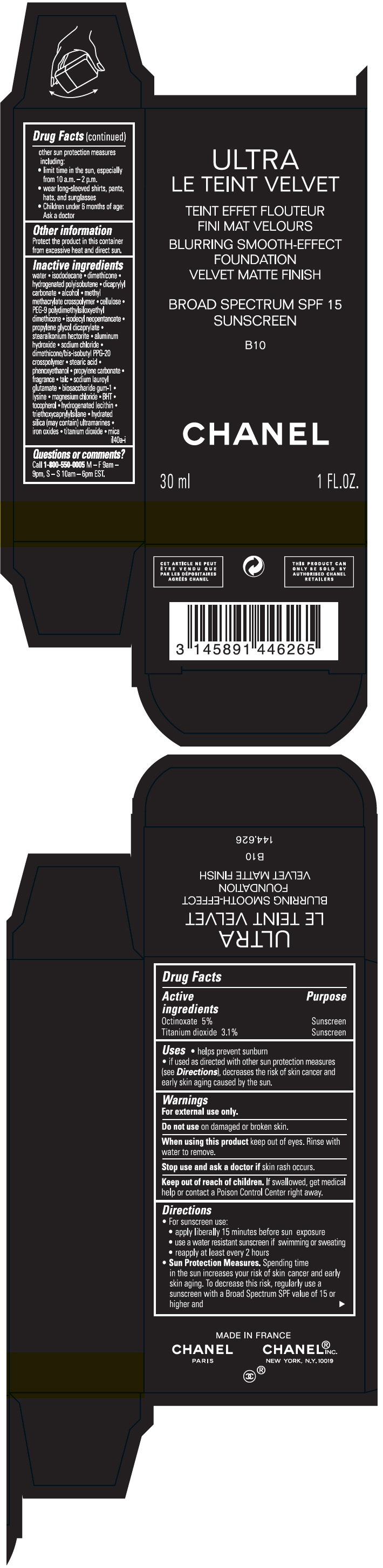
Label: ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN B10- octinoxate and titanium dioxide liquid
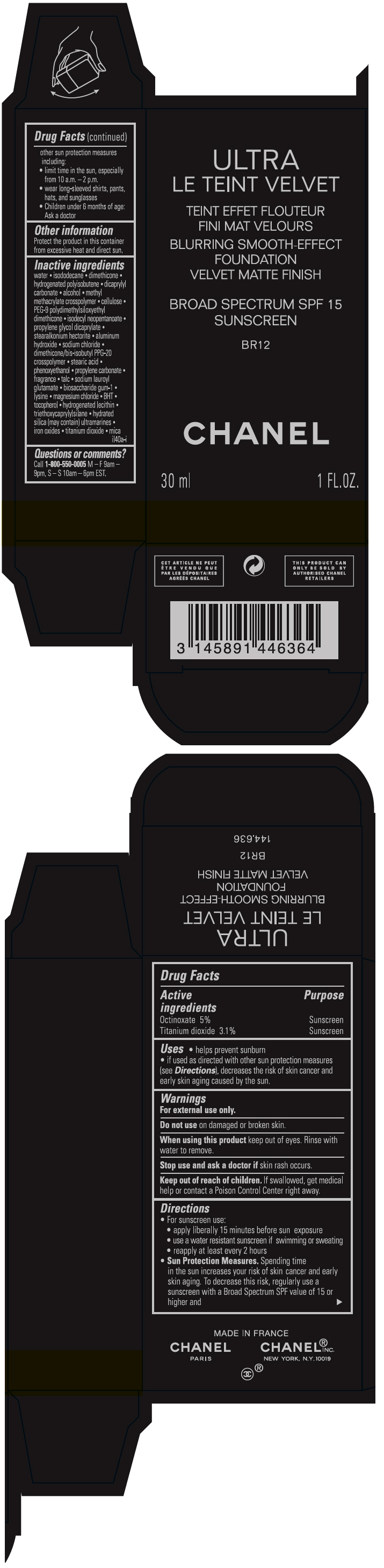
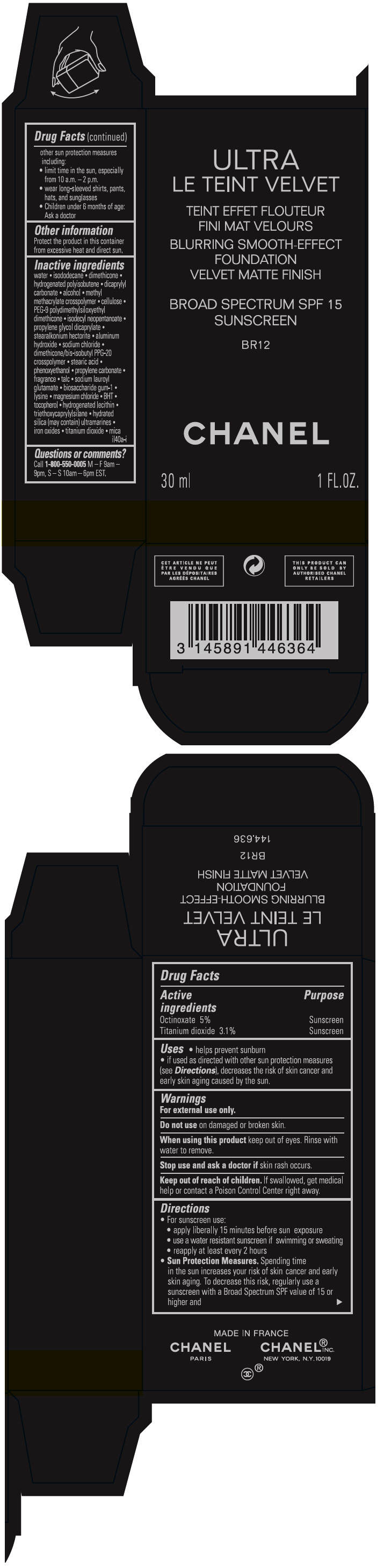
ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN BR12- octinoxate and titanium dioxide liquid
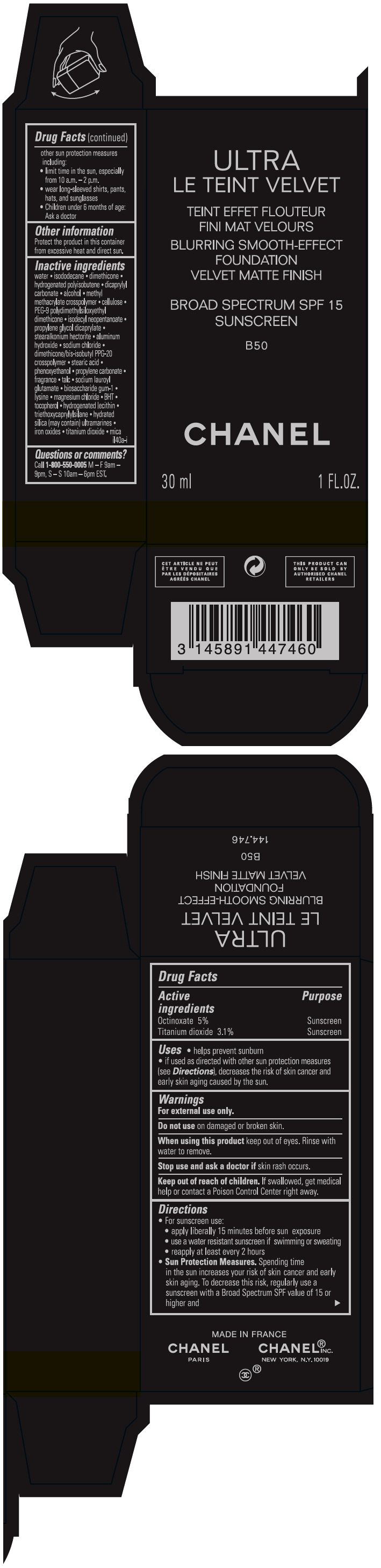
ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINIS .......SH BROAD SPECTRUM SPF 15 SUNSCREEN B50- octinoxate and titanium dioxide liquid
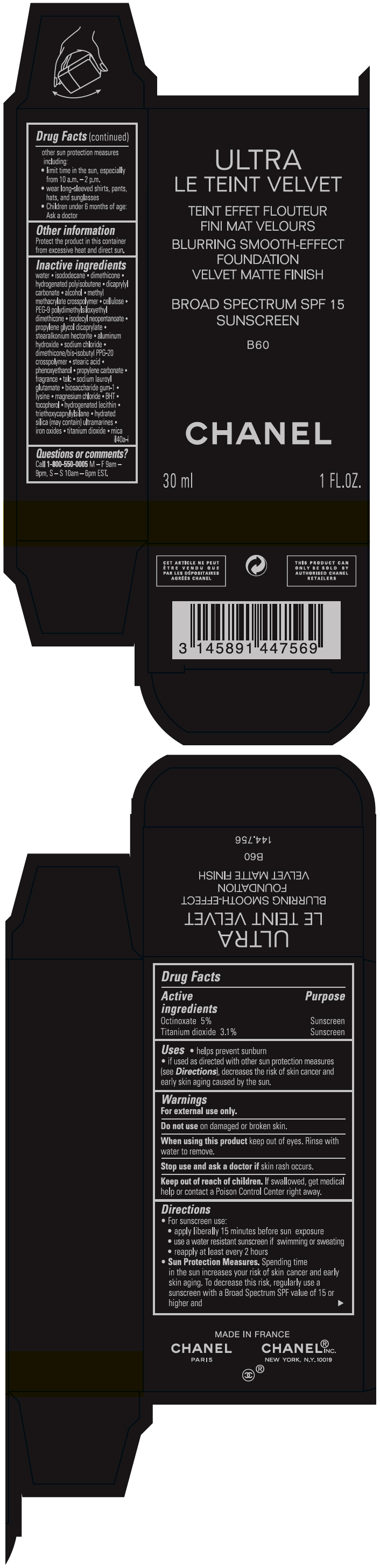
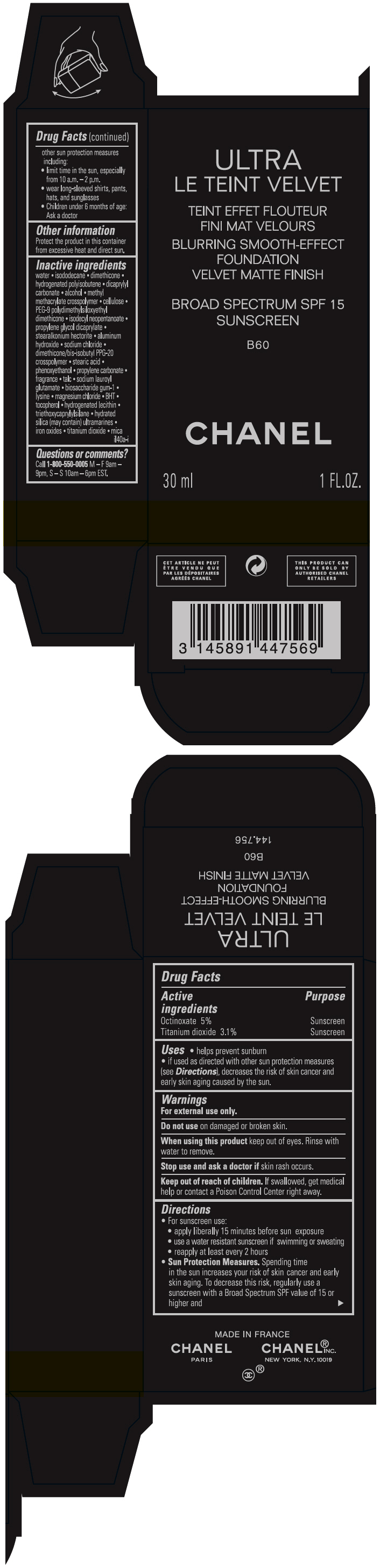
ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN B60- octinoxate and titanium dioxide liquid
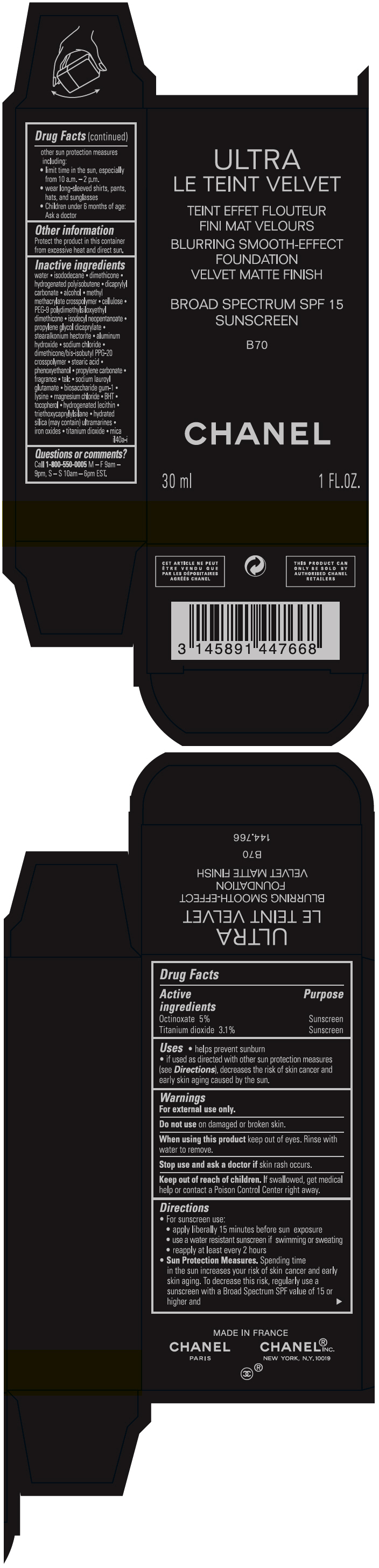
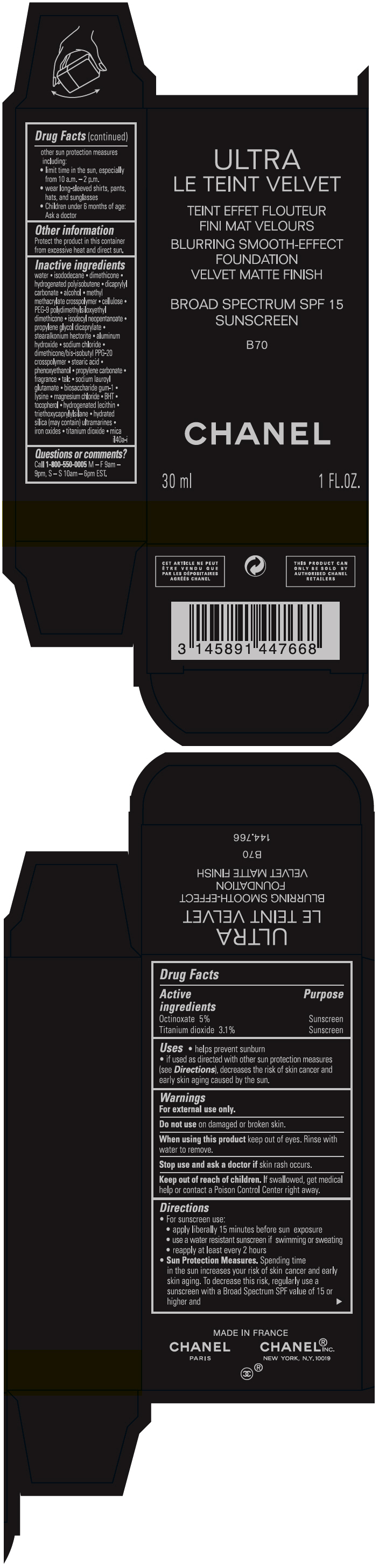
ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN B70- octinoxate and titanium dioxide liquid
-
NDC Code(s):
68745-2117-1,
68745-2117-2,
68745-2118-1,
68745-2118-2, view more68745-2119-1, 68745-2119-2, 68745-2120-1, 68745-2120-2, 68745-2121-1, 68745-2121-2, 68745-2122-1, 68745-2122-2, 68745-2123-1, 68745-2123-2, 68745-2124-1, 68745-2124-2, 68745-2125-1, 68745-2125-2, 68745-2126-1, 68745-2126-2, 68745-2127-1, 68745-2127-2
- Packager: CHANEL PARFUMS BEAUTE
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor
- For sunscreen use:
- Other information
-
Inactive ingredients
water • isododecane • dimethicone • hydrogenated polyisobutene • dicaprylyl carbonate • alcohol • methyl methacrylate crosspolymer • cellulose • PEG-9 polydimethylsiloxyethyl dimethicone • isodecyl neopentanoate • propylene glycol dicaprylate • stearalkonium hectorite • aluminum hydroxide • sodium chloride • dimethicone/bis-isobutyl PPG-20 crosspolymer • stearic acid • phenoxyethanol • propylene carbonate • fragrance • talc • sodium lauroyl glutamate • biosaccharide gum-1 • lysine • magnesium chloride • BHT • tocopherol • hydrogenated lecithin • triethoxycaprylylsilane • hydrated silica (may contain) ultramarines • iron oxides • titanium dioxide • mica
il40a-i
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton - B10
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton - BR12
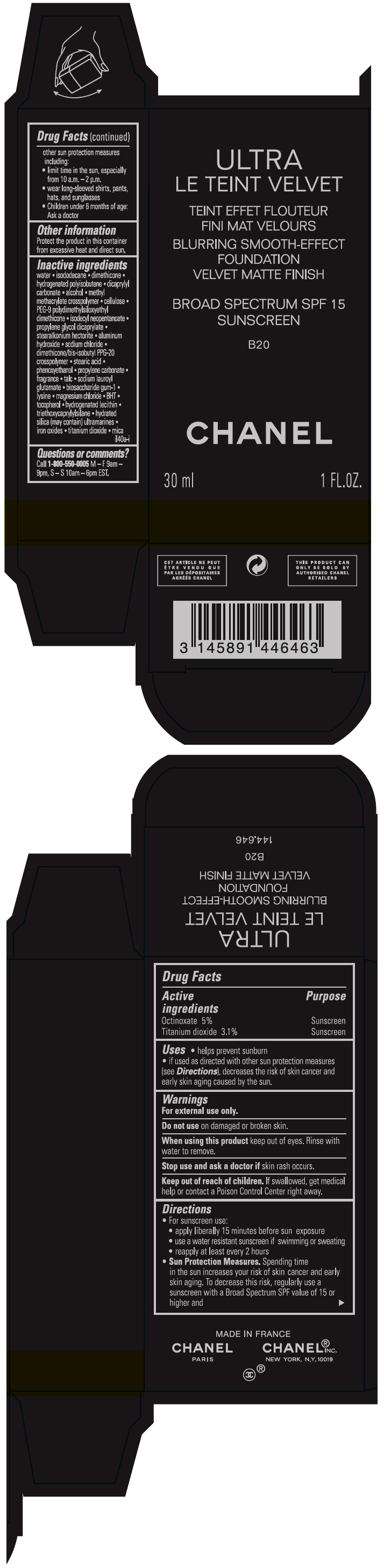
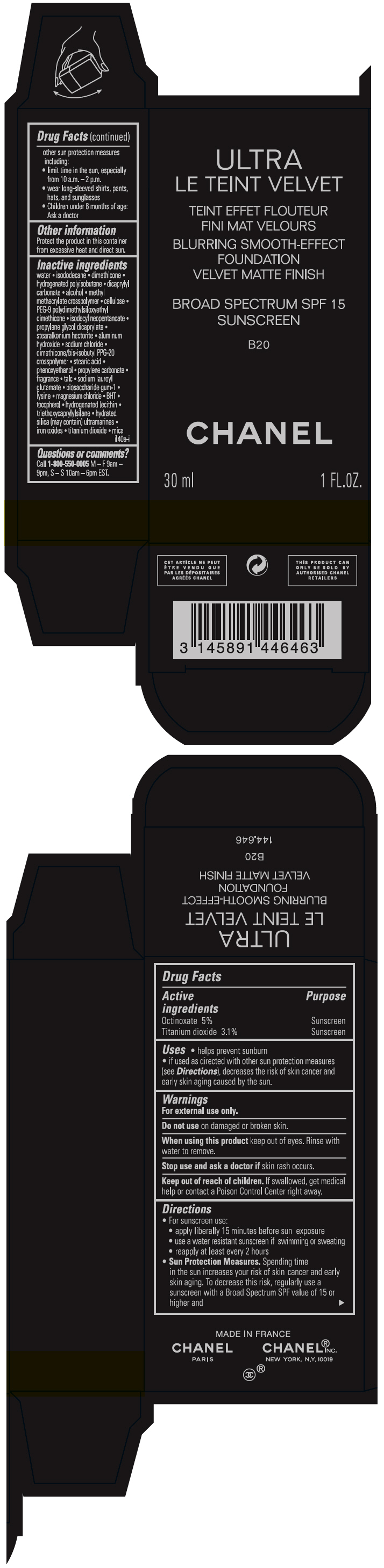
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton - B20
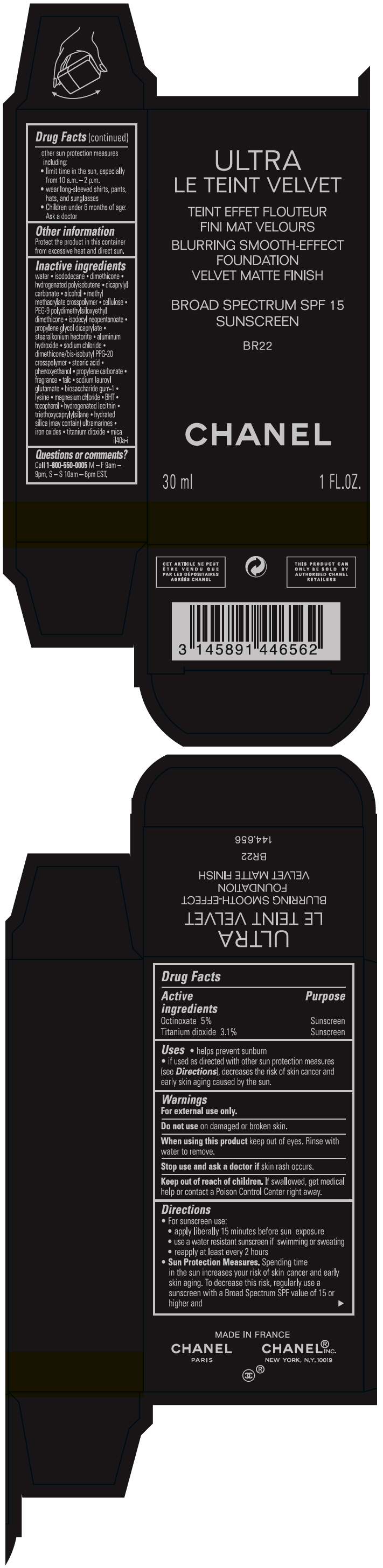
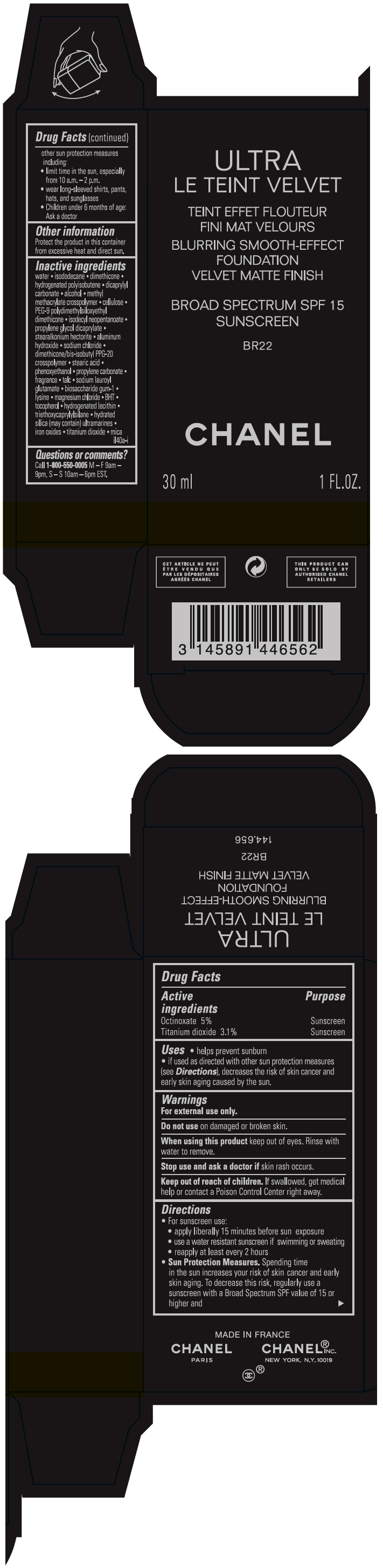
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton - BR22
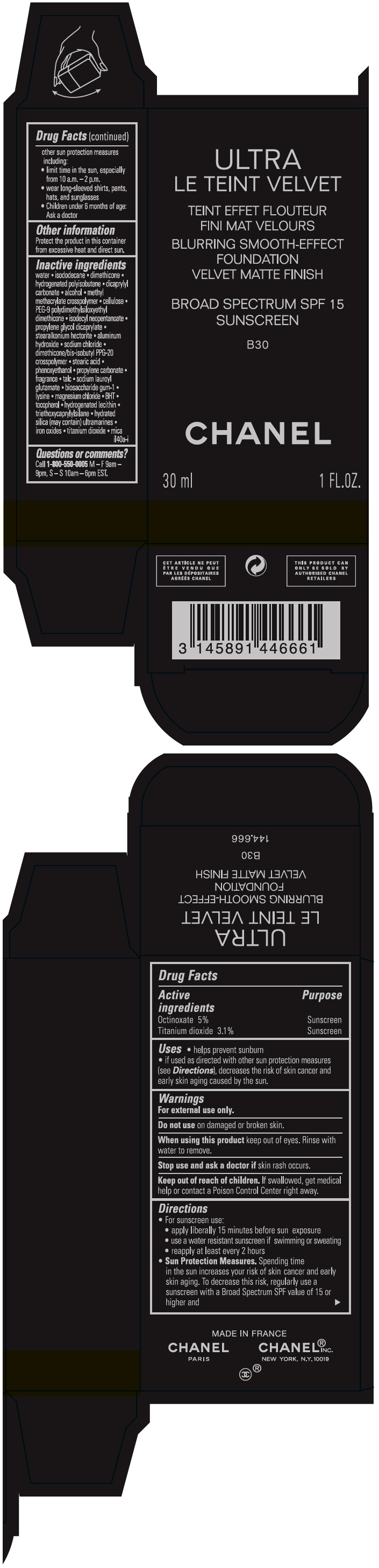
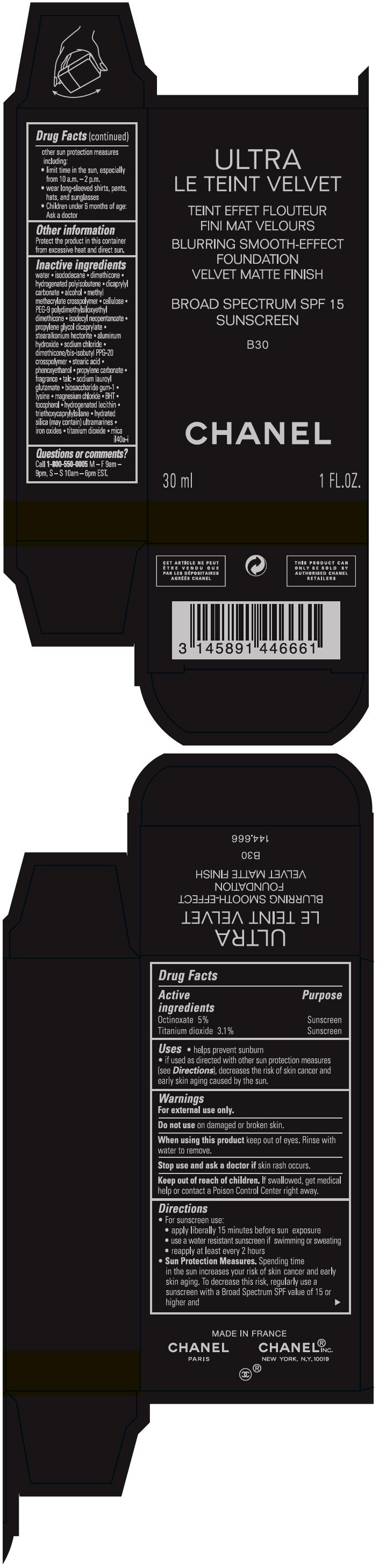
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton - B30
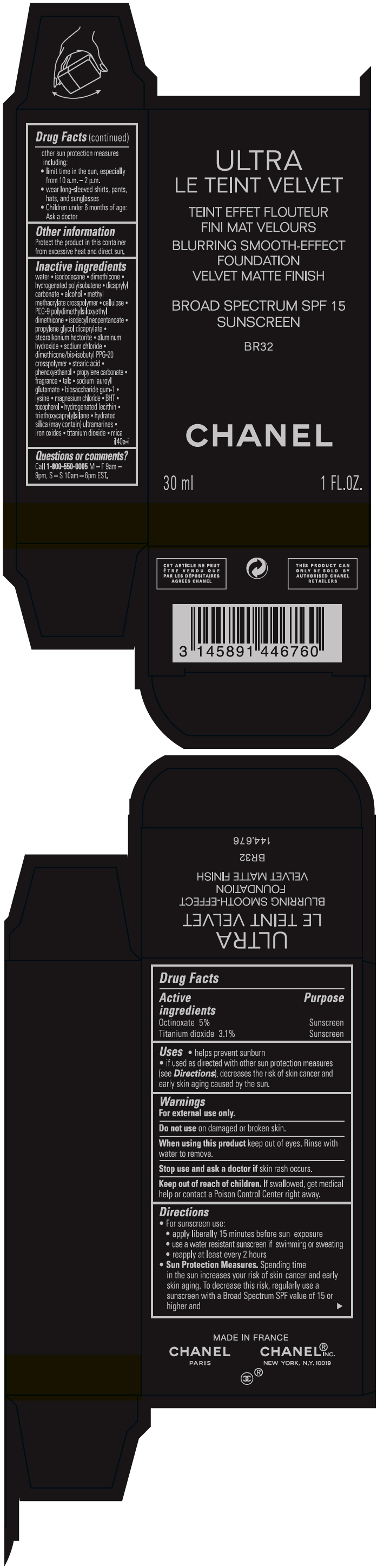
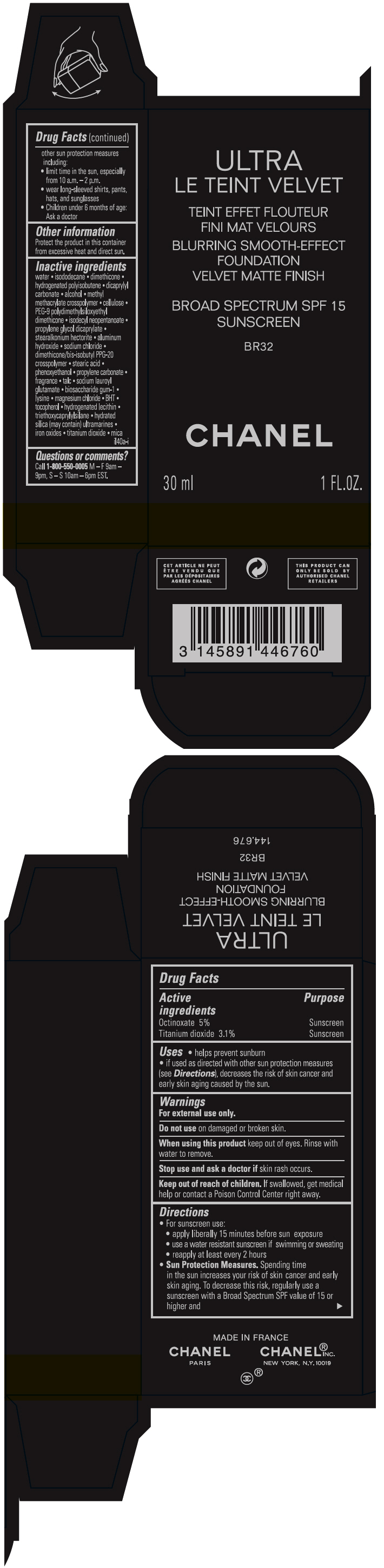
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton - BR32
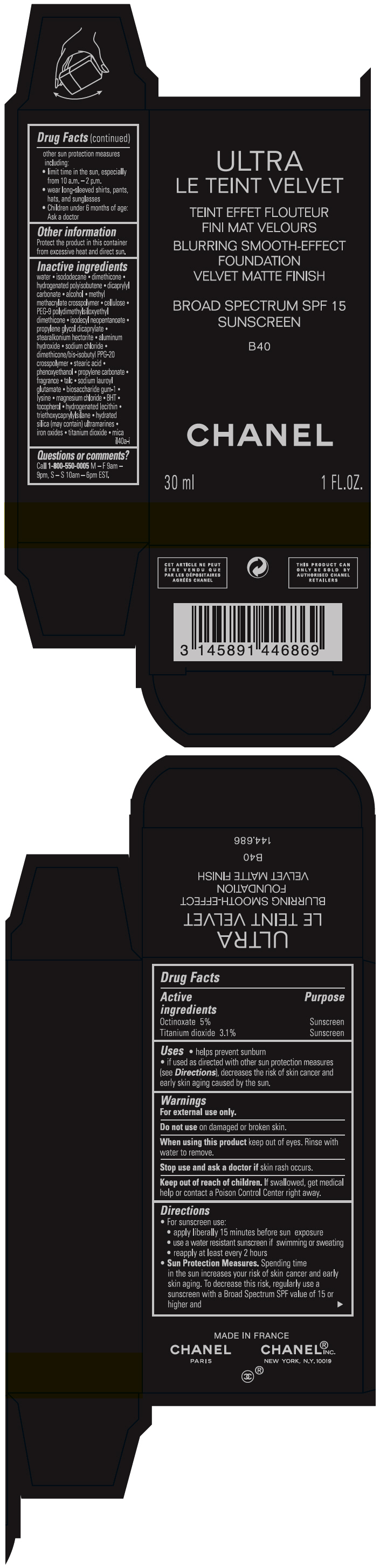
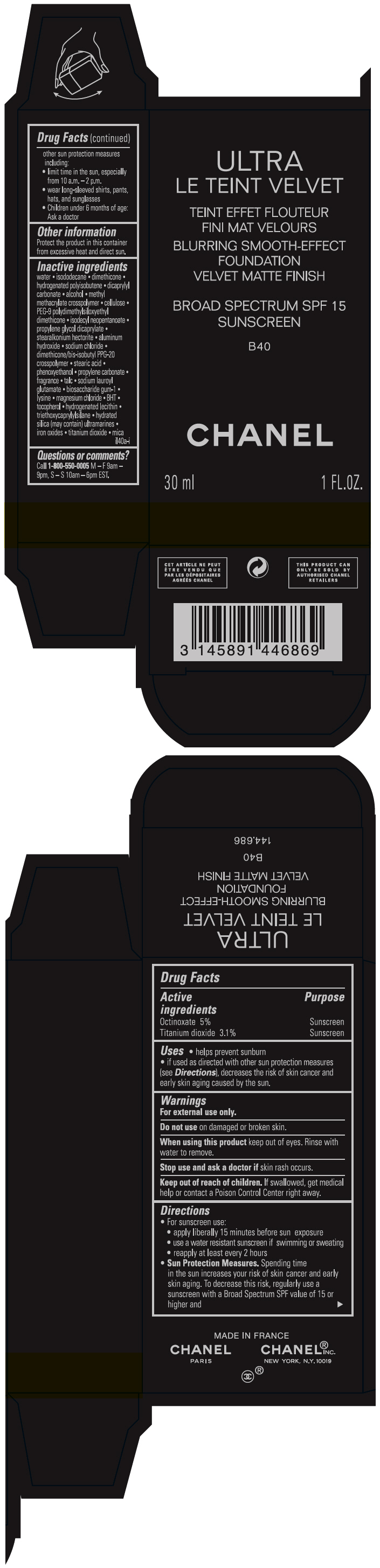
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton - B40
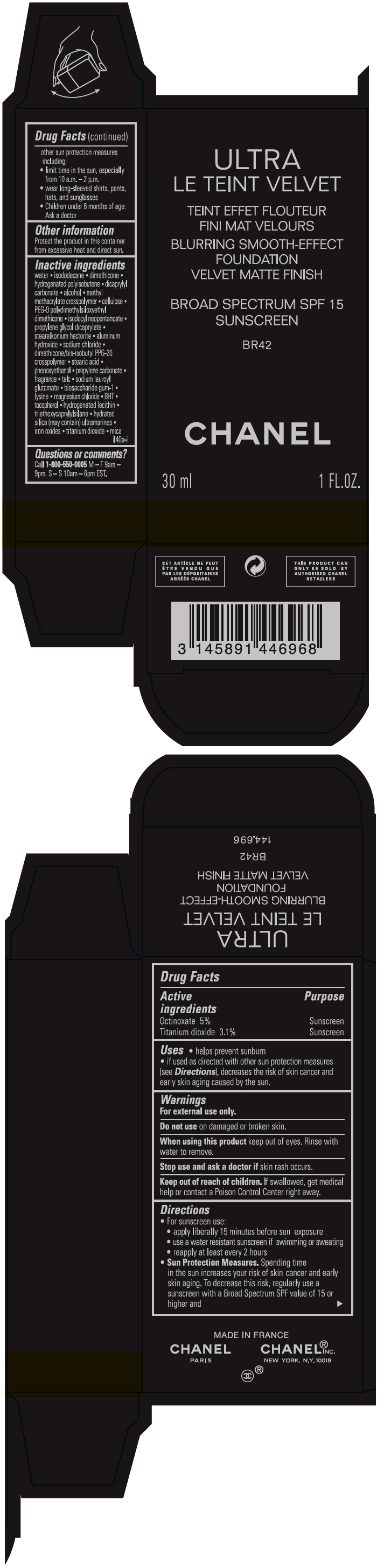
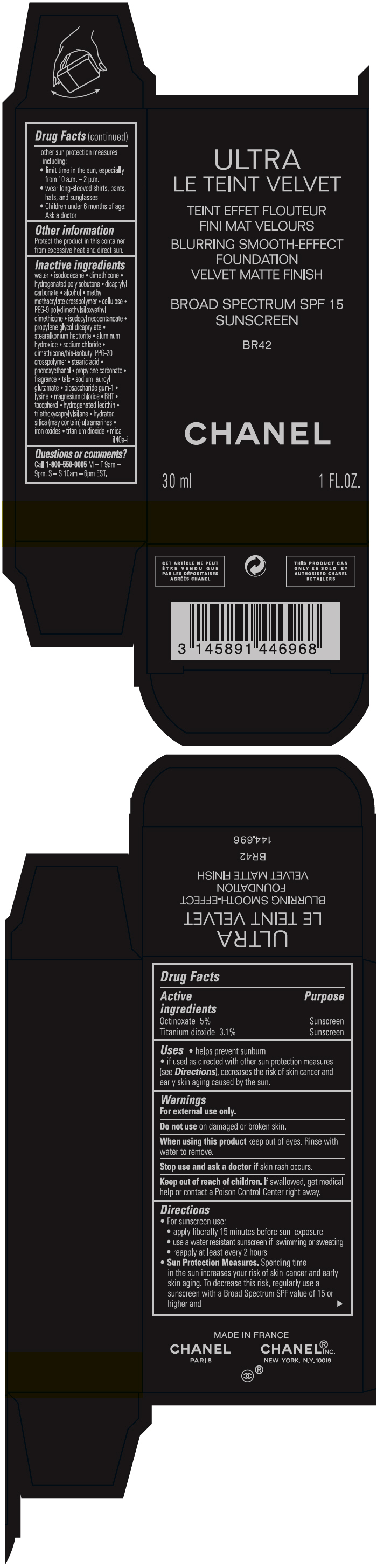
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton - BR42
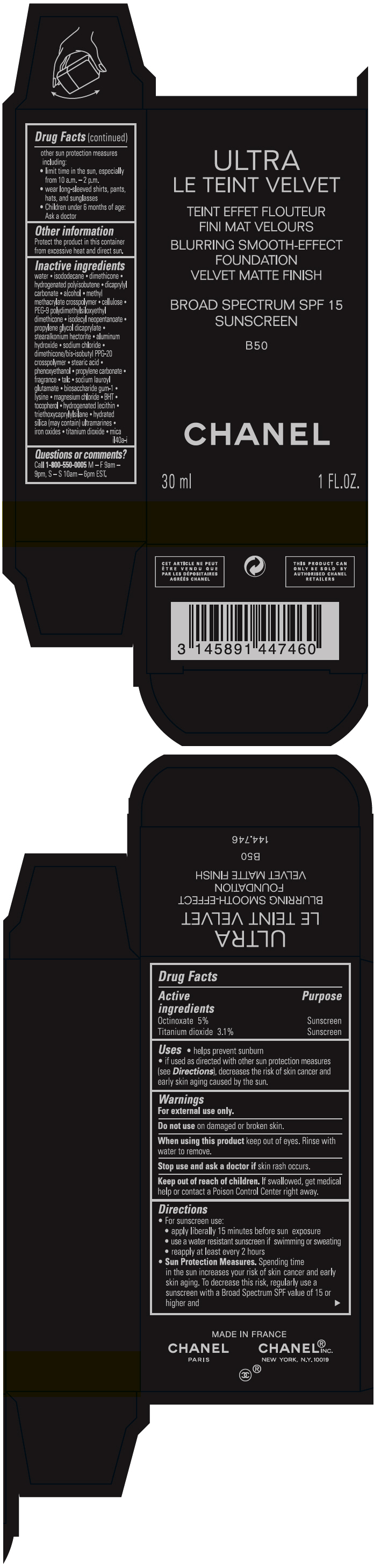
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton - B50
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton - B60
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton - B70
-
INGREDIENTS AND APPEARANCE
ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN B10
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68745-2117 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Titanium dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium dioxide 31 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) isododecane (UNII: A8289P68Y2) hydrogenated polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) dimethicone (UNII: 92RU3N3Y1O) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) powdered cellulose (UNII: SMD1X3XO9M) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) isodecyl neopentanoate (UNII: W60VYE24XC) propylene glycol dicaprylate (UNII: 581437HWX2) stearalkonium hectorite (UNII: OLX698AH5P) aluminum hydroxide (UNII: 5QB0T2IUN0) sodium chloride (UNII: 451W47IQ8X) dimethicone/bis-isobutyl PPG-20 crosspolymer (UNII: O4I3UFO6ZF) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) propylene carbonate (UNII: 8D08K3S51E) talc (UNII: 7SEV7J4R1U) sodium lauroyl glutamate (UNII: NCX1UU2D33) biosaccharide gum-1 (UNII: BB4PU4V09H) lysine (UNII: K3Z4F929H6) magnesium chloride (UNII: 02F3473H9O) butylated hydroxytoluene (UNII: 1P9D0Z171K) tocopherol (UNII: R0ZB2556P8) hydrogenated soybean lecithin (UNII: H1109Z9J4N) triethoxycaprylylsilane (UNII: LDC331P08E) hydrated silica (UNII: Y6O7T4G8P9) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68745-2117-1 1 in 1 CARTON 08/15/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68745-2117-2 1 in 1 CARTON 08/15/2019 2 20 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/15/2019 ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN BR12
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68745-2118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Titanium dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium dioxide 31 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) isododecane (UNII: A8289P68Y2) hydrogenated polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) dimethicone (UNII: 92RU3N3Y1O) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) powdered cellulose (UNII: SMD1X3XO9M) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) isodecyl neopentanoate (UNII: W60VYE24XC) propylene glycol dicaprylate (UNII: 581437HWX2) stearalkonium hectorite (UNII: OLX698AH5P) aluminum hydroxide (UNII: 5QB0T2IUN0) sodium chloride (UNII: 451W47IQ8X) dimethicone/bis-isobutyl PPG-20 crosspolymer (UNII: O4I3UFO6ZF) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) propylene carbonate (UNII: 8D08K3S51E) talc (UNII: 7SEV7J4R1U) sodium lauroyl glutamate (UNII: NCX1UU2D33) biosaccharide gum-1 (UNII: BB4PU4V09H) lysine (UNII: K3Z4F929H6) magnesium chloride (UNII: 02F3473H9O) butylated hydroxytoluene (UNII: 1P9D0Z171K) tocopherol (UNII: R0ZB2556P8) hydrogenated soybean lecithin (UNII: H1109Z9J4N) triethoxycaprylylsilane (UNII: LDC331P08E) hydrated silica (UNII: Y6O7T4G8P9) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68745-2118-1 1 in 1 CARTON 08/15/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68745-2118-2 1 in 1 CARTON 08/15/2019 2 20 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/15/2019 ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN B20
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68745-2119 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Titanium dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium dioxide 31 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) isododecane (UNII: A8289P68Y2) hydrogenated polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) dimethicone (UNII: 92RU3N3Y1O) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) powdered cellulose (UNII: SMD1X3XO9M) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) isodecyl neopentanoate (UNII: W60VYE24XC) propylene glycol dicaprylate (UNII: 581437HWX2) stearalkonium hectorite (UNII: OLX698AH5P) aluminum hydroxide (UNII: 5QB0T2IUN0) sodium chloride (UNII: 451W47IQ8X) dimethicone/bis-isobutyl PPG-20 crosspolymer (UNII: O4I3UFO6ZF) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) propylene carbonate (UNII: 8D08K3S51E) talc (UNII: 7SEV7J4R1U) sodium lauroyl glutamate (UNII: NCX1UU2D33) biosaccharide gum-1 (UNII: BB4PU4V09H) lysine (UNII: K3Z4F929H6) magnesium chloride (UNII: 02F3473H9O) butylated hydroxytoluene (UNII: 1P9D0Z171K) tocopherol (UNII: R0ZB2556P8) hydrogenated soybean lecithin (UNII: H1109Z9J4N) triethoxycaprylylsilane (UNII: LDC331P08E) hydrated silica (UNII: Y6O7T4G8P9) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68745-2119-1 1 in 1 CARTON 08/15/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68745-2119-2 1 in 1 CARTON 08/15/2019 2 20 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/15/2019 ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN BR22
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68745-2120 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Titanium dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium dioxide 31 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) isododecane (UNII: A8289P68Y2) hydrogenated polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) dimethicone (UNII: 92RU3N3Y1O) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) powdered cellulose (UNII: SMD1X3XO9M) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) isodecyl neopentanoate (UNII: W60VYE24XC) propylene glycol dicaprylate (UNII: 581437HWX2) stearalkonium hectorite (UNII: OLX698AH5P) aluminum hydroxide (UNII: 5QB0T2IUN0) sodium chloride (UNII: 451W47IQ8X) dimethicone/bis-isobutyl PPG-20 crosspolymer (UNII: O4I3UFO6ZF) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) propylene carbonate (UNII: 8D08K3S51E) talc (UNII: 7SEV7J4R1U) sodium lauroyl glutamate (UNII: NCX1UU2D33) biosaccharide gum-1 (UNII: BB4PU4V09H) lysine (UNII: K3Z4F929H6) magnesium chloride (UNII: 02F3473H9O) butylated hydroxytoluene (UNII: 1P9D0Z171K) tocopherol (UNII: R0ZB2556P8) hydrogenated soybean lecithin (UNII: H1109Z9J4N) triethoxycaprylylsilane (UNII: LDC331P08E) hydrated silica (UNII: Y6O7T4G8P9) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68745-2120-1 1 in 1 CARTON 08/15/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68745-2120-2 1 in 1 CARTON 08/15/2019 2 20 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/15/2019 ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN B30
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68745-2121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Titanium dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium dioxide 31 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) isododecane (UNII: A8289P68Y2) hydrogenated polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) dimethicone (UNII: 92RU3N3Y1O) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) powdered cellulose (UNII: SMD1X3XO9M) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) isodecyl neopentanoate (UNII: W60VYE24XC) propylene glycol dicaprylate (UNII: 581437HWX2) stearalkonium hectorite (UNII: OLX698AH5P) aluminum hydroxide (UNII: 5QB0T2IUN0) sodium chloride (UNII: 451W47IQ8X) dimethicone/bis-isobutyl PPG-20 crosspolymer (UNII: O4I3UFO6ZF) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) propylene carbonate (UNII: 8D08K3S51E) talc (UNII: 7SEV7J4R1U) sodium lauroyl glutamate (UNII: NCX1UU2D33) biosaccharide gum-1 (UNII: BB4PU4V09H) lysine (UNII: K3Z4F929H6) magnesium chloride (UNII: 02F3473H9O) butylated hydroxytoluene (UNII: 1P9D0Z171K) tocopherol (UNII: R0ZB2556P8) hydrogenated soybean lecithin (UNII: H1109Z9J4N) triethoxycaprylylsilane (UNII: LDC331P08E) hydrated silica (UNII: Y6O7T4G8P9) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68745-2121-1 1 in 1 CARTON 08/15/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68745-2121-2 1 in 1 CARTON 08/15/2019 2 20 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/15/2019 ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN BR32
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68745-2122 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Titanium dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium dioxide 31 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) isododecane (UNII: A8289P68Y2) hydrogenated polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) dimethicone (UNII: 92RU3N3Y1O) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) powdered cellulose (UNII: SMD1X3XO9M) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) isodecyl neopentanoate (UNII: W60VYE24XC) propylene glycol dicaprylate (UNII: 581437HWX2) stearalkonium hectorite (UNII: OLX698AH5P) aluminum hydroxide (UNII: 5QB0T2IUN0) sodium chloride (UNII: 451W47IQ8X) dimethicone/bis-isobutyl PPG-20 crosspolymer (UNII: O4I3UFO6ZF) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) propylene carbonate (UNII: 8D08K3S51E) talc (UNII: 7SEV7J4R1U) sodium lauroyl glutamate (UNII: NCX1UU2D33) biosaccharide gum-1 (UNII: BB4PU4V09H) lysine (UNII: K3Z4F929H6) magnesium chloride (UNII: 02F3473H9O) butylated hydroxytoluene (UNII: 1P9D0Z171K) tocopherol (UNII: R0ZB2556P8) hydrogenated soybean lecithin (UNII: H1109Z9J4N) triethoxycaprylylsilane (UNII: LDC331P08E) hydrated silica (UNII: Y6O7T4G8P9) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68745-2122-1 1 in 1 CARTON 08/15/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68745-2122-2 1 in 1 CARTON 08/15/2019 2 20 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/15/2019 ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN B40
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68745-2123 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Titanium dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium dioxide 31 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) isododecane (UNII: A8289P68Y2) hydrogenated polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) dimethicone (UNII: 92RU3N3Y1O) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) powdered cellulose (UNII: SMD1X3XO9M) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) isodecyl neopentanoate (UNII: W60VYE24XC) propylene glycol dicaprylate (UNII: 581437HWX2) stearalkonium hectorite (UNII: OLX698AH5P) aluminum hydroxide (UNII: 5QB0T2IUN0) sodium chloride (UNII: 451W47IQ8X) dimethicone/bis-isobutyl PPG-20 crosspolymer (UNII: O4I3UFO6ZF) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) propylene carbonate (UNII: 8D08K3S51E) talc (UNII: 7SEV7J4R1U) sodium lauroyl glutamate (UNII: NCX1UU2D33) biosaccharide gum-1 (UNII: BB4PU4V09H) lysine (UNII: K3Z4F929H6) magnesium chloride (UNII: 02F3473H9O) butylated hydroxytoluene (UNII: 1P9D0Z171K) tocopherol (UNII: R0ZB2556P8) hydrogenated soybean lecithin (UNII: H1109Z9J4N) triethoxycaprylylsilane (UNII: LDC331P08E) hydrated silica (UNII: Y6O7T4G8P9) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68745-2123-1 1 in 1 CARTON 08/15/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68745-2123-2 1 in 1 CARTON 08/15/2019 2 20 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/15/2019 ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN BR42
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68745-2124 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Titanium dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium dioxide 31 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) isododecane (UNII: A8289P68Y2) hydrogenated polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) dimethicone (UNII: 92RU3N3Y1O) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) powdered cellulose (UNII: SMD1X3XO9M) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) isodecyl neopentanoate (UNII: W60VYE24XC) propylene glycol dicaprylate (UNII: 581437HWX2) stearalkonium hectorite (UNII: OLX698AH5P) aluminum hydroxide (UNII: 5QB0T2IUN0) sodium chloride (UNII: 451W47IQ8X) dimethicone/bis-isobutyl PPG-20 crosspolymer (UNII: O4I3UFO6ZF) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) propylene carbonate (UNII: 8D08K3S51E) talc (UNII: 7SEV7J4R1U) sodium lauroyl glutamate (UNII: NCX1UU2D33) biosaccharide gum-1 (UNII: BB4PU4V09H) lysine (UNII: K3Z4F929H6) magnesium chloride (UNII: 02F3473H9O) butylated hydroxytoluene (UNII: 1P9D0Z171K) tocopherol (UNII: R0ZB2556P8) hydrogenated soybean lecithin (UNII: H1109Z9J4N) triethoxycaprylylsilane (UNII: LDC331P08E) hydrated silica (UNII: Y6O7T4G8P9) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68745-2124-1 1 in 1 CARTON 08/15/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68745-2124-2 1 in 1 CARTON 08/15/2019 2 20 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/15/2019 ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN B50
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68745-2125 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Titanium dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium dioxide 31 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) isododecane (UNII: A8289P68Y2) hydrogenated polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) dimethicone (UNII: 92RU3N3Y1O) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) powdered cellulose (UNII: SMD1X3XO9M) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) isodecyl neopentanoate (UNII: W60VYE24XC) propylene glycol dicaprylate (UNII: 581437HWX2) stearalkonium hectorite (UNII: OLX698AH5P) aluminum hydroxide (UNII: 5QB0T2IUN0) sodium chloride (UNII: 451W47IQ8X) dimethicone/bis-isobutyl PPG-20 crosspolymer (UNII: O4I3UFO6ZF) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) propylene carbonate (UNII: 8D08K3S51E) talc (UNII: 7SEV7J4R1U) sodium lauroyl glutamate (UNII: NCX1UU2D33) biosaccharide gum-1 (UNII: BB4PU4V09H) lysine (UNII: K3Z4F929H6) magnesium chloride (UNII: 02F3473H9O) butylated hydroxytoluene (UNII: 1P9D0Z171K) tocopherol (UNII: R0ZB2556P8) hydrogenated soybean lecithin (UNII: H1109Z9J4N) triethoxycaprylylsilane (UNII: LDC331P08E) hydrated silica (UNII: Y6O7T4G8P9) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68745-2125-1 1 in 1 CARTON 08/15/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68745-2125-2 1 in 1 CARTON 08/15/2019 2 20 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/15/2019 ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN B60
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68745-2126 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Titanium dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium dioxide 31 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) isododecane (UNII: A8289P68Y2) hydrogenated polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) dimethicone (UNII: 92RU3N3Y1O) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) powdered cellulose (UNII: SMD1X3XO9M) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) isodecyl neopentanoate (UNII: W60VYE24XC) propylene glycol dicaprylate (UNII: 581437HWX2) stearalkonium hectorite (UNII: OLX698AH5P) aluminum hydroxide (UNII: 5QB0T2IUN0) sodium chloride (UNII: 451W47IQ8X) dimethicone/bis-isobutyl PPG-20 crosspolymer (UNII: O4I3UFO6ZF) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) propylene carbonate (UNII: 8D08K3S51E) talc (UNII: 7SEV7J4R1U) sodium lauroyl glutamate (UNII: NCX1UU2D33) biosaccharide gum-1 (UNII: BB4PU4V09H) lysine (UNII: K3Z4F929H6) magnesium chloride (UNII: 02F3473H9O) butylated hydroxytoluene (UNII: 1P9D0Z171K) tocopherol (UNII: R0ZB2556P8) hydrogenated soybean lecithin (UNII: H1109Z9J4N) triethoxycaprylylsilane (UNII: LDC331P08E) hydrated silica (UNII: Y6O7T4G8P9) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68745-2126-1 1 in 1 CARTON 08/15/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68745-2126-2 1 in 1 CARTON 08/15/2019 2 20 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/15/2019 ULTRA LE TEINT VELVET BLURRING SMOOTH-EFFECT FOUNDATION VELVET MATTE FINISH BROAD SPECTRUM SPF 15 SUNSCREEN B70
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68745-2127 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 mg in 1 mL Titanium dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium dioxide 31 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) isododecane (UNII: A8289P68Y2) hydrogenated polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) dimethicone (UNII: 92RU3N3Y1O) dicaprylyl carbonate (UNII: 609A3V1SUA) alcohol (UNII: 3K9958V90M) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) powdered cellulose (UNII: SMD1X3XO9M) PEG-9 polydimethylsiloxyethyl dimethicone (UNII: TYP81E471F) isodecyl neopentanoate (UNII: W60VYE24XC) propylene glycol dicaprylate (UNII: 581437HWX2) stearalkonium hectorite (UNII: OLX698AH5P) aluminum hydroxide (UNII: 5QB0T2IUN0) sodium chloride (UNII: 451W47IQ8X) dimethicone/bis-isobutyl PPG-20 crosspolymer (UNII: O4I3UFO6ZF) stearic acid (UNII: 4ELV7Z65AP) phenoxyethanol (UNII: HIE492ZZ3T) propylene carbonate (UNII: 8D08K3S51E) talc (UNII: 7SEV7J4R1U) sodium lauroyl glutamate (UNII: NCX1UU2D33) biosaccharide gum-1 (UNII: BB4PU4V09H) lysine (UNII: K3Z4F929H6) magnesium chloride (UNII: 02F3473H9O) butylated hydroxytoluene (UNII: 1P9D0Z171K) tocopherol (UNII: R0ZB2556P8) hydrogenated soybean lecithin (UNII: H1109Z9J4N) triethoxycaprylylsilane (UNII: LDC331P08E) hydrated silica (UNII: Y6O7T4G8P9) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68745-2127-1 1 in 1 CARTON 08/15/2019 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68745-2127-2 1 in 1 CARTON 08/15/2019 2 20 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/15/2019 Labeler - CHANEL PARFUMS BEAUTE (275137669) Establishment Name Address ID/FEI Business Operations CHANEL PARFUMS BEAUTE 277032509 MANUFACTURE(68745-2117, 68745-2118, 68745-2119, 68745-2120, 68745-2121, 68745-2122, 68745-2123, 68745-2124, 68745-2125, 68745-2126, 68745-2127) , LABEL(68745-2117, 68745-2118, 68745-2119, 68745-2120, 68745-2121, 68745-2122, 68745-2123, 68745-2124, 68745-2125, 68745-2126, 68745-2127)