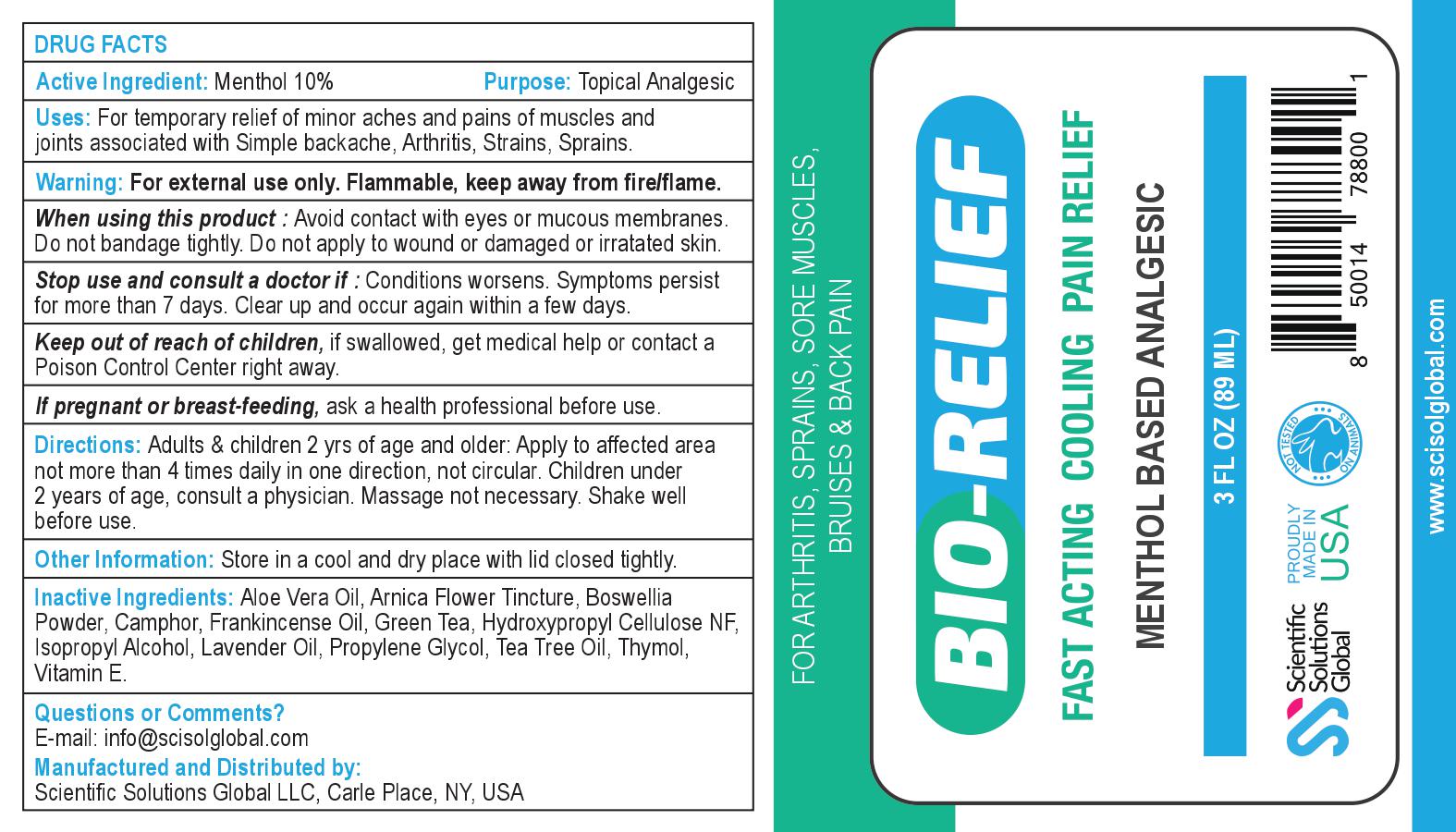
Label: BIO-RELIEF- menthol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 71718-200-01 - Packager: Scientific Solutions Global LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 11, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
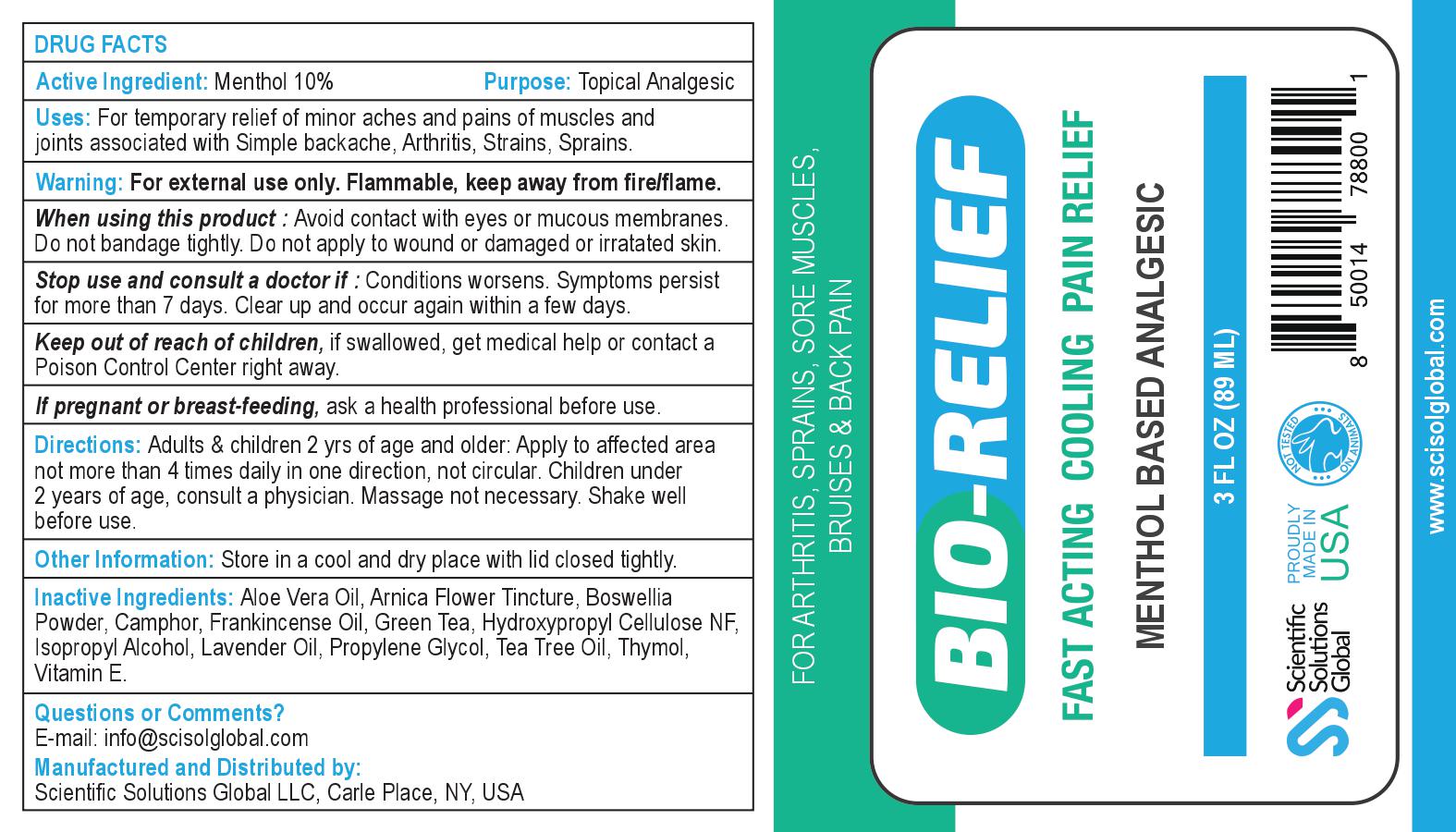
For temporary relief of minor aches and pains of muscles and joints associated with • simple backache • arthritis • strains• bruises • sprains
Keep out of reach of children, if swallowed, get medical help or contact a Poison Control Center right away.
Adults & children 2 yrs of age and older: Apply to affected area not more than 4 times daily in one direction, not circular. Children under 2 years of age, consult a physician. Massage not necessary. Shake well before use.
-
INGREDIENTS AND APPEARANCE
BIO-RELIEF
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71718-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 8.9 g in 89 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) LAVENDER OIL (UNII: ZBP1YXW0H8) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) ARNICA MONTANA (UNII: O80TY208ZW) ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) TEA TREE OIL (UNII: VIF565UC2G) ALOE VERA LEAF (UNII: ZY81Z83H0X) THYMOL (UNII: 3J50XA376E) Product Characteristics Color white (Opaque gel) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71718-200-01 89 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2020 Labeler - Scientific Solutions Global LLC (097291290) Registrant - Scientific Solutions Global LLC (097291290) Establishment Name Address ID/FEI Business Operations Scientific Solutions Global LLC 097291290 manufacture(71718-200)