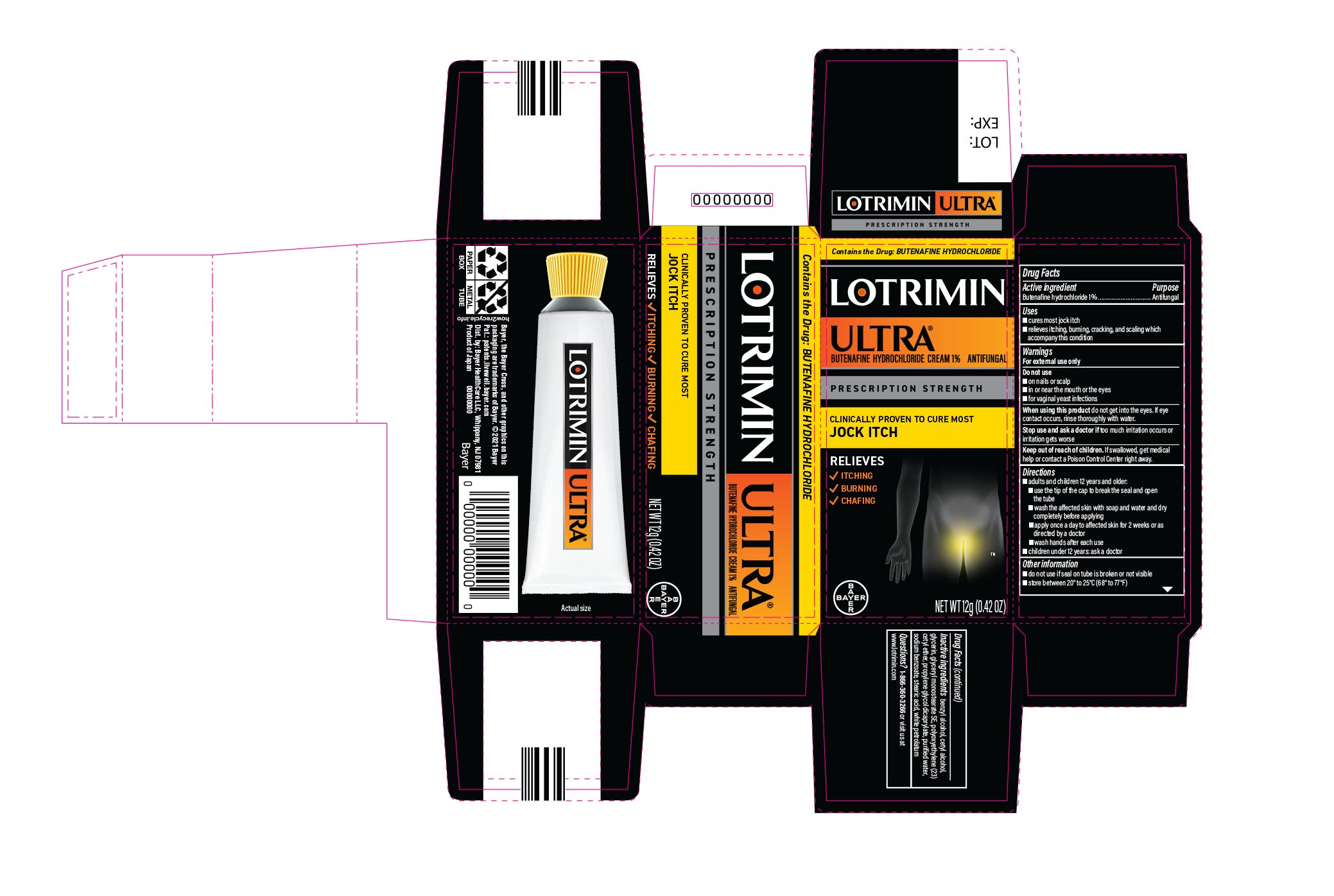
Label: LOTRIMIN ULTRA JOCK ITCH- butenafine hydrochloride cream
- NDC Code(s): 11523-4339-1, 11523-4339-2, 11523-4339-3
- Packager: Bayer Healthcare LLC.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated September 26, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- adults and children 12 years and over:
- use the tip of the cap to break the seal and open the tube
- wash the affected skin with soap and water and dry completely before applying
- apply once a day to affected skin for 2 weeks or as directed by a doctor
- wash hands after each use
- children under 12 years: ask a doctor
- adults and children 12 years and over:
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 12 g Tube Carton
-
INGREDIENTS AND APPEARANCE
LOTRIMIN ULTRA JOCK ITCH
butenafine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-4339 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUTENAFINE HYDROCHLORIDE (UNII: R8XA2029ZI) (BUTENAFINE - UNII:91Y494NL0X) BUTENAFINE HYDROCHLORIDE 1 g in 100 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) CETETH-23 (UNII: 495CTZ441V) PROPYLENE GLYCOL DICAPRYLATE (UNII: 581437HWX2) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) PETROLATUM (UNII: 4T6H12BN9U) BENZYL ALCOHOL (UNII: LKG8494WBH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) Product Characteristics Color white (white to off white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-4339-1 1 in 1 CARTON 02/22/2002 1 12 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11523-4339-2 1 in 1 CARTON 02/22/2002 2 15 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11523-4339-3 1 in 1 CARTON 09/26/2023 3 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021307 02/22/2002 Labeler - Bayer Healthcare LLC. (112117283)