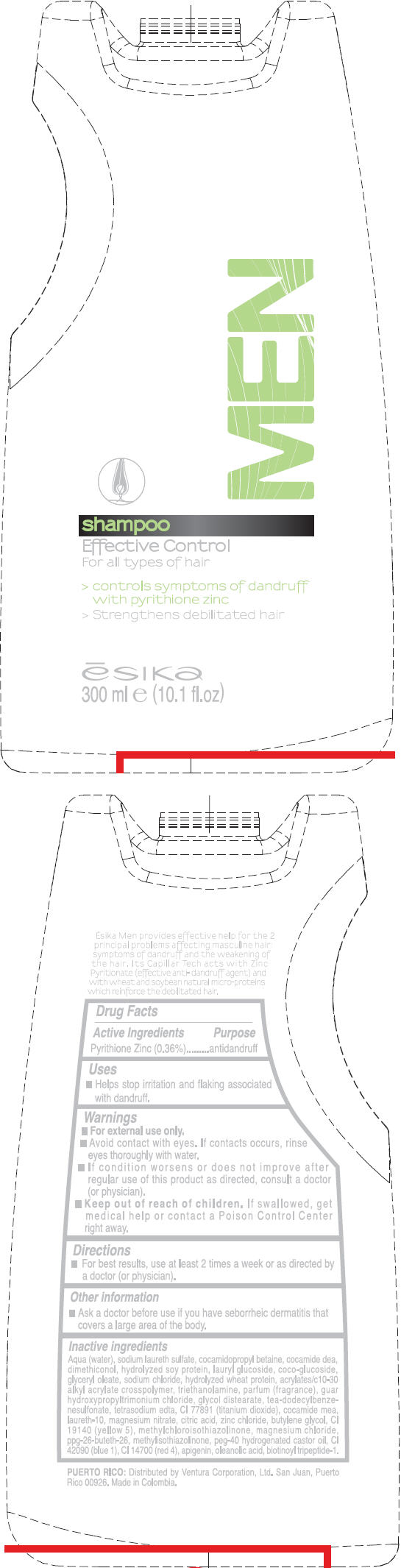
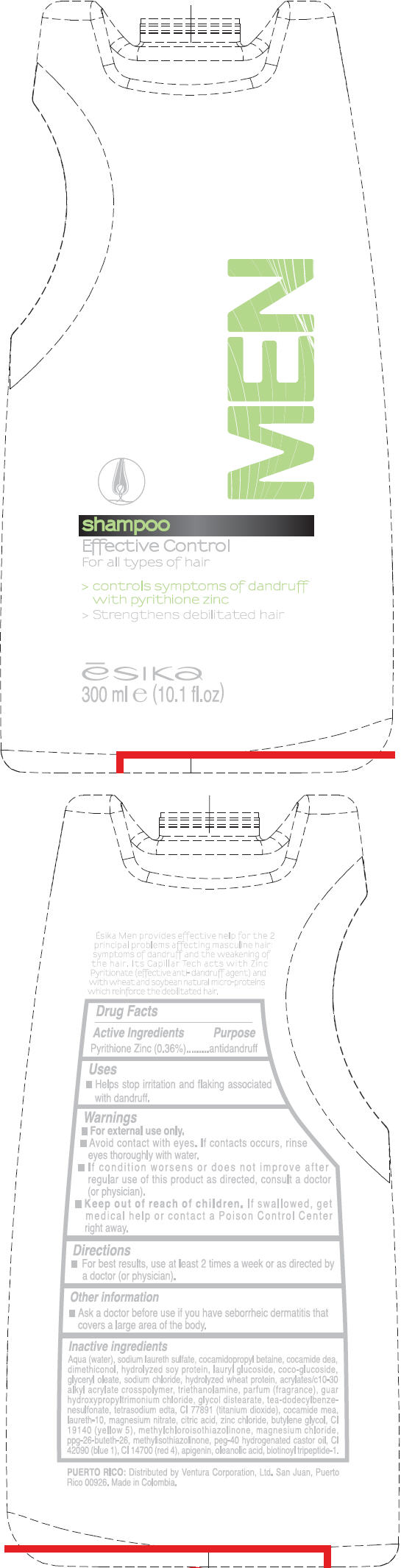
Label: ESIKA- pyrithione zinc liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-341-01 - Packager: Ventura Corporation (San Juan, P.R)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 18, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aqua (water), sodium laureth sulfate, cocamidopropyl betaine, cocamide dea, dimethiconol , hydrolyzed soy protein, lauryl glucoside, coco-glucoside , glyceryl oleate, sodium chloride, hydrolyzed wheat protein, acrylates/c10-30 alkyl acrylate crosspolymer, triethanolamine, fragrance, guar hydroxypropyltrimonium chloride, glycol distearate, tea-dodecylbenzenesulfonate, tetrasodium edta, ci 77891 (titanium dioxide ), cocamide mea, laureth-10 , magnesium nitrate, citric acid, zinc chloride, butylene glycol, ci 19140 (yellow 5), methylchloroisothiazolinone , magnesium chloride, ppg-26-buteth-26, methylisothiazolinone, peg-40 hydrogenated castor oil, ci 42090 (blue 1), ci 14700 (red 4), apigenin, oleanolic acid, biotinoyl tripeptide-1.
- PRINCIPAL DISPLAY PANEL - 300 ml Bottle Label
-
INGREDIENTS AND APPEARANCE
ESIKA MEN EFFECTIVE CONTROL
pyrithione zinc liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-341 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pyrithione Zinc (UNII: R953O2RHZ5) (Pyrithione Zinc - UNII:R953O2RHZ5) Pyrithione Zinc 0.0036 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) sodium laureth sulfate (UNII: BPV390UAP0) cocamidopropyl betaine (UNII: 5OCF3O11KX) coco diethanolamide (UNII: 92005F972D) lauryl glucoside (UNII: 76LN7P7UCU) coco glucoside (UNII: ICS790225B) glyceryl monooleate (UNII: 4PC054V79P) sodium chloride (UNII: 451W47IQ8X) trolamine (UNII: 9O3K93S3TK) glycol distearate (UNII: 13W7MDN21W) tea-dodecylbenzenesulfonate (UNII: 8HM7ZD48HN) edetate sodium (UNII: MP1J8420LU) titanium dioxide (UNII: 15FIX9V2JP) coco monoethanolamide (UNII: C80684146D) laureth-10 (UNII: BD7AST04GA) magnesium nitrate (UNII: 77CBG3UN78) citric acid monohydrate (UNII: 2968PHW8QP) zinc chloride (UNII: 86Q357L16B) butylene glycol (UNII: 3XUS85K0RA) fd&c yellow no. 5 (UNII: I753WB2F1M) methylchloroisothiazolinone (UNII: DEL7T5QRPN) magnesium chloride (UNII: 02F3473H9O) methylisothiazolinone (UNII: 229D0E1QFA) polyoxyl 40 hydrogenated castor oil (UNII: 7YC686GQ8F) fd&c blue no. 1 (UNII: H3R47K3TBD) fd&c red no. 4 (UNII: X3W0AM1JLX) oleanolic acid (UNII: 6SMK8R7TGJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-341-01 300 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part358H 05/17/2011 Labeler - Ventura Corporation (San Juan, P.R) (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE