Label: NOON POST PROCEDURE PROGRAM- allantoin, titanium dioxide, octocrylene, and avobenzone kit
- NDC Code(s): 78863-1230-2, 78863-1270-2, 78863-2600-1
- Packager: Noon Aesthetics M.R. Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive Ingredients
Water, Propylene Glycol, Glycerin, Dimethyl Sulfone, Strontium Chloride Hexahydrate, Dimethicone, Cetyl Alcohol, Glyceryl Stearate, Propanediol, Dipotassium Glycyrrhizate, Polysorbate 20, PEG-40 Stearate, Laureth-4, Porphyridium Cruentum Extract, Arnica Montana Flower Extract, sh-Oligopeptide-1 (EGF), sh-Oligopeptide-2 (IGF-1), sh-Polypeptide-1 (bFGF), sh-Polypeptide-9 (VEGF), sh-Polypeptide-11 (FGF-1), Bacillus/Folic Acid Ferment Filtrate Extract, Octyldodecyl Xyloside, Butylene Glycol, Sodium Hyaluronate, 1,2-Hexanediol, Acetyl Glutamine, Octyldodecanol, PEG-30 Dipolyhydroxystearate, Sorbitan Tristearate, Lecithin, Phenoxyethanol, Benzyl Alcohol, Caprylyl Glycol, Potassium Sorbate, Disodium EDTA, Sodium Benzoate.
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure and as needed.
- Use a water-resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
-
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad- spectrum SPF value of 15 or higher and other sun protection measures including: - limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses.
- Other Information
-
Inactive Ingredients
Water, Isononyl Isononanoate, Cyclopentasiloxane, Isohexadecane, Glyceryl Stearate, PEG 100 Stearate, Glycerin, Silica, Potassium Cetyl Phosphate, Sorbitol, Sorbitan Stearate, Magnesium Aluminum Silicate, Xanthan Gum, Sodium Phytate, Xylitol, Nicotinamide, Butyrospermum Parkii (Shea ) Butter, Tocopheryl Acetate, Ascorbyl Tetraisopalmitate, Ectoin, Phenoxyethanol, Anhydroxylitol, Stearic Acid, Alumina, PPG-3 Benzyl Ether Myristate, PVP, Xylitylglucoside, Citrus Aurantium Bergamia fruit oil, Fragrance, Sodium Polyacrylate, Ethylhexyl Cocoate, Caprylyl Glycol, Alcohol.
- SPL UNCLASSIFIED SECTION
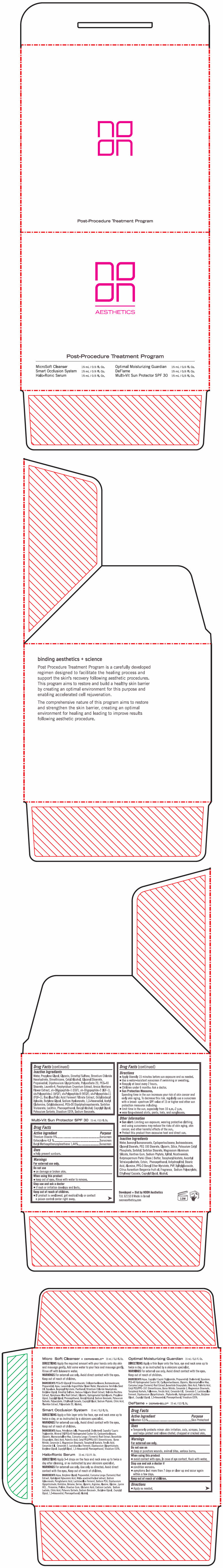
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
NOON POST PROCEDURE PROGRAM
allantoin, titanium dioxide, octocrylene, and avobenzone kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78863-2600 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78863-2600-1 1 in 1 CARTON 10/01/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 15 mL Part 2 1 BOTTLE 15 mL Part 3 1 BOTTLE 15 mL Part 4 1 BOTTLE 15 mL Part 5 1 BOTTLE 15 mL Part 6 1 BOTTLE 15 mL Part 1 of 6 MICRO SOFT CLEANSER
cleansing (cold creams, cleansing lotions, liquids, and pads) creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR PEG-20 GLYCERYL TRIISOSTEARATE (UNII: 4714JOJ107) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR ISOHEXADECANE (UNII: 918X1OUF1E) INGR PROPANEDIOL (UNII: 5965N8W85T) INGR WATER (UNII: 059QF0KO0R) INGR MACADAMIA OIL (UNII: 515610SU8C) INGR SQUALANE (UNII: GW89575KF9) INGR ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) INGR PANTHENOL (UNII: WV9CM0O67Z) INGR STRONTIUM CHLORIDE HEXAHYDRATE (UNII: O09USB7Z44) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) INGR NYMPHAEA ALBA ROOT (UNII: 866B05KWBR) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR PROPYLENE GLYCOL (UNII: 6DC9Q167V3) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR BENZYL ALCOHOL (UNII: LKG8494WBH) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR ETHYLHEXYL COCOATE (UNII: I1MPW273QS) INGR PHYTATE SODIUM (UNII: 88496G1ERL) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 10/01/2023 Part 2 of 6 SMART OCCLUSION SYSTEM
face and neck (excluding shaving preparations) creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR PROPANEDIOL (UNII: 5965N8W85T) INGR CHOLESTEROL (UNII: 97C5T2UQ7J) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR MINERAL OIL (UNII: T5L8T28FGP) INGR POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) INGR TURMERIC (UNII: 856YO1Z64F) INGR ISOSORBIDE DICAPRYLATE (UNII: 0IK29C4889) INGR OLEIC ACID (UNII: 2UMI9U37CP) INGR PALMITIC ACID (UNII: 2V16EO95H1) INGR BORON NITRIDE (UNII: 2U4T60A6YD) INGR CERAMIDE NP (UNII: 4370DF050B) INGR MAGNESIUM STEARATE (UNII: 70097M6I30) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR FERULIC ACID (UNII: AVM951ZWST) INGR CERAMIDE AP (UNII: F1X8L2B00J) INGR CERAMIDE NG (UNII: C04977SRJ5) INGR GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR 1,2-HEXANEDIOL (UNII: TR046Y3K1G) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR EDETATE TRISODIUM (UNII: 420IP921MB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 10/01/2023 Part 3 of 6 HALO-RONIC SERUM
face and neck (excluding shaving preparations) creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR PROPANEDIOL (UNII: 5965N8W85T) INGR TURMERIC (UNII: 856YO1Z64F) INGR AZADIRACHTA INDICA LEAF (UNII: HKY915780T) INGR HYALURONATE SODIUM (UNII: YSE9PPT4TH) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) INGR HISTIDINE (UNII: 4QD397987E) INGR BETAINE (UNII: 3SCV180C9W) INGR SERINE (UNII: 452VLY9402) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR ARGININE (UNII: 94ZLA3W45F) INGR ALANINE (UNII: OF5P57N2ZX) INGR GLYCINE (UNII: TE7660XO1C) INGR LYSINE HYDROCHLORIDE (UNII: JNJ23Q2COM) INGR THREONINE (UNII: 2ZD004190S) INGR PROLINE (UNII: 9DLQ4CIU6V) INGR GLUTAMIC ACID (UNII: 3KX376GY7L) INGR CALCIUM LACTATE ANHYDROUS (UNII: 2URQ2N32W3) INGR SODIUM LACTATE (UNII: TU7HW0W0QT) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR 1,2-HEXANEDIOL (UNII: TR046Y3K1G) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 10/01/2023 Part 4 of 6 OPTIMAL MOISTURIZING GUARDIAN
moisturizing creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR PROPANEDIOL (UNII: 5965N8W85T) INGR CHOLESTEROL (UNII: 97C5T2UQ7J) INGR SQUALANE (UNII: GW89575KF9) INGR POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) INGR TURMERIC (UNII: 856YO1Z64F) INGR ISOSORBIDE DICAPRYLATE (UNII: 0IK29C4889) INGR OLEIC ACID (UNII: 2UMI9U37CP) INGR PALMITIC ACID (UNII: 2V16EO95H1) INGR BORON NITRIDE (UNII: 2U4T60A6YD) INGR CERAMIDE NP (UNII: 4370DF050B) INGR MAGNESIUM STEARATE (UNII: 70097M6I30) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR BUCKMINSTERFULLERENE (UNII: NP9U26B839) INGR FERULIC ACID (UNII: AVM951ZWST) INGR CERAMIDE AP (UNII: F1X8L2B00J) INGR CERAMIDE NG (UNII: C04977SRJ5) INGR GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR 1,2-HEXANEDIOL (UNII: TR046Y3K1G) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR EDETATE TRISODIUM (UNII: 420IP921MB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 10/01/2023 Part 5 of 6 DEFLAME
allantoin creamProduct Information Item Code (Source) NDC:78863-1270 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) STRONTIUM CHLORIDE HEXAHYDRATE (UNII: O09USB7Z44) DIMETHICONE (UNII: 92RU3N3Y1O) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PROPANEDIOL (UNII: 5965N8W85T) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) POLYSORBATE 20 (UNII: 7T1F30V5YH) LAURETH-4 (UNII: 6HQ855798J) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) OCTYLDODECYL XYLOSIDE (UNII: 8Z6VNR46QM) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HYALURONATE SODIUM (UNII: YSE9PPT4TH) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ACEGLUTAMIDE (UNII: 01J18G9G97) OCTYLDODECANOL (UNII: 461N1O614Y) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) SORBITAN TRISTEARATE (UNII: 6LUM696811) PHENOXYETHANOL (UNII: HIE492ZZ3T) BENZYL ALCOHOL (UNII: LKG8494WBH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78863-1270-2 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 10/01/2023 Part 6 of 6 MULTI-VIT SUN PROTECTOR SPF 30
titanium dioxide, octocrylene, and avobenzone creamProduct Information Item Code (Source) NDC:78863-1230 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 48 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 14.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ISOHEXADECANE (UNII: 918X1OUF1E) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) SORBITOL (UNII: 506T60A25R) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) XANTHAN GUM (UNII: TTV12P4NEE) PHYTATE SODIUM (UNII: 88496G1ERL) XYLITOL (UNII: VCQ006KQ1E) NIACINAMIDE (UNII: 25X51I8RD4) SHEA BUTTER (UNII: K49155WL9Y) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ASCORBYL TETRAISOPALMITATE (UNII: 47143LT58A) ECTOINE (UNII: 7GXZ3858RY) PHENOXYETHANOL (UNII: HIE492ZZ3T) ANHYDROXYLITOL (UNII: 8XWR7NN42F) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) PPG-3 BENZYL ETHER MYRISTATE (UNII: 8075L58MKO) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) XYLITYLGLUCOSIDE (UNII: O0IEZ166FB) ETHYLHEXYL COCOATE (UNII: I1MPW273QS) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78863-1230-2 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 10/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M016 10/01/2023 Labeler - Noon Aesthetics M.R. Ltd (600185560) Establishment Name Address ID/FEI Business Operations Noon Aesthetics M.R. Ltd 600185560 MANUFACTURE(78863-2600)