Label: ARTICADENT- articaine hydrochloride and epinephrine bitartrate injection, solution
- NDC Code(s): 66312-601-16, 66312-602-16
- Packager: Dentsply Pharmaceutical
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated March 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ARTICADENT safely and effectively. See full prescribing information for ARTICADENT.
Articadent® (articaine hydrochloride and epinephrine injection), for intraoral submucosal infiltration use
Initial U.S. Approval: 2000RECENT MAJOR CHANGES
WARNINGS AND PRECAUTIONS, Methemoglobinemia (5.4)
INDICATIONS AND USAGE
ARTICADENT is a combination of articaine HCl, an amide local anesthetic, and epinephrine, a vasoconstrictor, is indicated for local, infiltrative, or conductive anesthesia in both simple and complex dental procedures in adults and pediatric patients 4 years of age or older. (1)
DOSAGE AND ADMINISTRATION
- For dental procedures by intraoral submucosal infiltration or nerve block. (2.1)
- -
- For infiltration: 0.5-2.5 mL (20-100 mg articaine HCl)
- -
- For nerve block: 0.5-3.4 mL (20-136 mg articaine HCl)
- -
- For oral surgery: 1.0-5.1 mL (40-204 mg articaine HCl)
- For most routine dental procedures, ARTICADENT containing epinephrine 1:200,000 is preferred. However, when more pronounced hemostasis or improved visualization of the surgical field are required, ARTICADENT containing epinephrine 1:100,000 may be used. (2.1)
- Maximum recommended dosages (2.2):
- -
- Healthy adults: 7 mg/kg of articaine HCl and 0.0017 mg/kg of epinephrine (equivalent to 0.175 mL/kg for either product presentation, articaine HCl and epinephrine 1:100,000 or 1:200,000)
- -
- Pediatric patients: 4-16 years: 7 mg/kg of articaine HCl and 0.0017 mg/kg of epinephrine (equivalent to 0.175 mL/kg for either product presentation, articaine HCl and epinephrine 1:100,000 or 1:200,000)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Known hypersensitivity to sulfite. (4)
WARNINGS AND PRECAUTIONS
Accidental Intravascular Injection: May be associated with convulsions followed by coma and respiratory arrest. Resuscitative equipment, oxygen and other resuscitative drugs should be available. (5.1)
Systemic Toxicity: Systemic absorption of ARTICADENT can produce effects on the central nervous and cardiovascular systems. (5.2)
Vasoconstrictor Toxicity: Local anesthetic solutions like ARTICADENT that contain a vasoconstrictor should be used cautiously, especially in patients with impaired cardiovascular function or vascular disease. (5.3)
Methemoglobinemia: Cases of methemoglobinemia have been reported in association with local anesthetic use. (5.4)
ADVERSE REACTIONS
The most common adverse reactions (incidence >2%) are headache and pain. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact DENTSPLY Pharmaceutical at 1-800-989-8826 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on animal studies, may cause fetal harm. (8.1)
Nursing Mothers: Exercise caution when administering to a nursing woman. (8.3)
Pediatric Use: Safety and effectiveness in pediatric patients below the age of 4 years have not been established. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2024
- For dental procedures by intraoral submucosal infiltration or nerve block. (2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
2.2 Maximum Recommended Dosages
2.3 Dosage in Specific Populations
2.4 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Accidental Intravascular Injection
5.2 Systemic Toxicity
5.3 Vasoconstrictor Toxicity
5.4 Methemoglobinemia
5.5 Anaphylaxis and Allergic-Type Reactions
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal and Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
Table 1 summarizes the recommended dosages of ARTICADENT administered by intraoral submucosal infiltration or nerve block for various types of anesthetic dental procedures in healthy adults and pediatric patients.
Table 1: Recommended Dosages for Both Strengths Procedure ARTICADENT Injection Volume (mL) Total dose of articaine HCl (mg) Infiltration 0.5 mL to 2.5 mL 20 mg to 100 mg Nerve block 0.5 mL to 3.4 mL 20 mg to 136 mg Oral surgery 1 mL to 5.1 mL 40 mg to 204 mg The recommended dosages of ARTICADENT in healthy adults serve only as a guide to the amount of anesthetic required for most routine dental procedures. The dosages to be used in adults depend on several factors such as type and extent of surgical procedure, depth of anesthesia, degree of muscular relaxation, and condition of the patient. In all cases, administer the lowest dosage that will produce the desired result.
The dosages of ARTICADENT to be used in pediatric patients aged 4 to 16 years old are determined by the age and weight of the patient and the type of dental procedure. For most routine dental procedures, ARTICADENT containing epinephrine 1:200,000 is preferred. However, when more pronounced hemostasis or improved visualization of the surgical field are required, ARTICADENT containing epinephrine 1:100,000 may be used.
The onset of anesthesia and the duration of anesthesia are proportional to the dosage of the local anesthetic used. Exercise caution when employing large volumes because the incidence of adverse reactions may be dose-related.
2.2 Maximum Recommended Dosages
- Healthy Adults: The maximum recommended dosage of ARTICADENT is 7 mg/kg of articaine and 0.0017 mg/kg of epinephrine (equivalent to 0.175 mL/kg for either product presentation, articaine HCl and 1:100,000 or 1:200,000 epinephrine).
- Pediatric Patients Ages 4 to 16 Years: The maximum recommended dosage of ARTICADENT is 7 mg/kg of articaine and 0.0017 mg/kg of epinephrine (equivalent to 0.175 mL/kg for either product presentation, articaine HCl and 1:100,000 or 1:200,000 epinephrine) [see Use in Specific Populations (8.4)].
2.3 Dosage in Specific Populations
Lower dosages or dosage reduction may be required in debilitated patients, acutely ill patients, elderly patients, and pediatric patients commensurate with their age and physical condition. No studies have been performed in patients with renal or liver impairment. Exercise caution when using ARTICADENT in patients with severe liver disease. [see Warnings and Precautions (5.2), Use in Specific Populations (8.4, 8.5, and 8.6)].
2.4 Important Administration Instructions
Visually inspect ARTICADENT for particulate matter and discoloration prior to administration.
Prior to using the glass cartridges, disinfect by wiping the cap thoroughly with USP grade isopropyl alcohol (70%). Avoid use of isopropyl alcohol, as well as solutions of ethyl alcohol that are not of USP grade because they may contain denaturants that are injurious to rubber. Immersion is not recommended. Discard unused portion.
-
3 DOSAGE FORMS AND STRENGTHS
Injection (clear, colorless solution), provided in:
- Glass cartridges (single-dose) containing (less than a full cartridge or more than one cartridge may be used for an individual patient):
- -
- Articaine hydrochloride 4% (40 mg/mL) and epinephrine 1:200,000 (as epinephrine bitartrate 0.009 mg/mL)
- -
- Articaine hydrochloride 4% (40 mg/mL) and epinephrine 1:100,000 (as epinephrine bitartrate 0.018 mg/mL)
- Glass cartridges (single-dose) containing (less than a full cartridge or more than one cartridge may be used for an individual patient):
-
4 CONTRAINDICATIONS
ARTICADENT is contraindicated in patients who are hypersensitive to products containing sulfites. Products containing sulfites may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people [see Warnings and Precautions (5.5)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Accidental Intravascular Injection
Accidental intravascular injection of ARTICADENT may be associated with convulsions, followed by central nervous system or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest. Dental practitioners who employ local anesthetic agents including ARTICADENT should be well versed in diagnosis and management of emergencies that may arise from their use. Resuscitative equipment, oxygen, and other resuscitative drugs should be available for immediate use. To avoid intravascular injection, aspiration should be performed before ARTICADENT is injected. The needle must be repositioned until no return of blood can be elicited by aspiration. Note, however, that the absence of blood in the syringe does not guarantee that intravascular injection has been avoided.
Small doses of local anesthetics injected in dental blocks may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. Confusion, convulsions, respiratory depression or respiratory arrest, and cardiovascular stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. Patients receiving these blocks should be observed constantly. Resuscitative equipment and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded [see Dosage and Administration (2.1)].
5.2 Systemic Toxicity
This includes toxicity arising from accidental intravascular injection of ARTICADENT discussed in Section 5.1, as well as that related to higher systemic concentrations of local anesthetics or epinephrine [see Warnings and Precautions (5.3)]. Systemic absorption of local anesthetics including ARTICADENT can produce effects on the central nervous and cardiovascular systems.
At blood concentrations achieved with therapeutic doses of ARTICADENT, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance are minimal. However, toxic blood concentrations of ARTICADENT can depress cardiac conduction and excitability, which may lead to atrioventricular block, ventricular arrhythmias, and cardiac arrest, possibly resulting in fatalities. In addition, myocardial contractility is depressed and peripheral vasodilatation occurs, leading to decreased cardiac output and arterial blood pressure. ARTICADENT should also be used with caution in patients with heart block as well as those with impaired cardiovascular function since they may be less able to compensate for functional changes associated with the prolongation of A-V conduction produced by these drugs.
Restlessness, anxiety, tinnitus, dizziness, blurred vision, tremors, depression, or drowsiness may be early warning signs of central nervous system toxicity.
Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient's state of consciousness should be performed after each local anesthetic injection of ARTICADENT. Repeated doses of ARTICADENT may cause significant increases in blood levels because of possible accumulation of the drug or its metabolites.
The lowest dosage that results in effective anesthesia should be used to decrease the risk of high plasma levels and serious adverse effects. Tolerance to elevated blood levels varies with the status of the patient. Resuscitative equipment, oxygen, and other resuscitative drugs should be available for immediate use. Precautions for epinephrine administration, discussed in Section 5.3, should be observed.
Debilitated patients, elderly patients, acutely ill patients, and pediatric patients should be given reduced doses commensurate with their age and physical condition [see Dosage and Administration (2.1, 2.3)]. No studies have been performed in patients with liver impairment, and caution should be used in patients with severe hepatic disease.
5.3 Vasoconstrictor Toxicity
ARTICADENT contains epinephrine, a vasoconstrictor that can cause local or systemic toxicity and should be used cautiously. Local toxicity may include ischemic injury or necrosis, which may be related to vascular spasm. ARTICADENT should be used with caution in patients during and following the administration of potent general anesthetic agents, since cardiac arrhythmias may occur under such conditions. Patients with peripheral vascular disease and those with hypertensive vascular disease may exhibit exaggerated vasoconstrictor response.
The American Heart Association has made the following recommendation regarding the use of local anesthetics with vasoconstrictors in patients with ischemic heart disease: "Vasoconstrictor agents should be used in local anesthesia solutions during dental practice only when it is clear that the procedure will be shortened or the analgesia rendered more profound. When a vasoconstrictor is indicated, extreme care should be taken to avoid intravascular injection. The minimum possible amount of vasoconstrictor should be used." (Kaplan, 1986). It is essential to aspirate before any injection to avoid administration of the drug into the blood stream.
5.4 Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure and are characterized by a cyanotic skin discoloration, and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue ARTICADENT and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
5.5 Anaphylaxis and Allergic-Type Reactions
ARTICADENT contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
-
6 ADVERSE REACTIONS
Reactions to articaine are characteristic of those associated with other amide-type local anesthetics. Adverse reactions to this group of drugs may also result from excessive plasma levels (which may be due to overdosage, unintentional intravascular injection, or slow metabolic degradation), injection technique, volume of injection, or hypersensitivity or they may be idiosyncratic.
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The reported adverse reactions are derived from clinical trials in the United States and the United Kingdom. Table 2 displays the adverse reactions reported in clinical trials where 882 individuals were exposed to ARTICADENT containing epinephrine 1:100,000. Table 3 displays the adverse reactions reported in clinical trials where 182 individuals were exposed to ARTICADENT containing epinephrine 1:100,000 and 179 individuals were exposed to ARTICADENT containing epinephrine 1:200,000.
Adverse reactions observed in at least 1% of patients:
Table 2: Adverse Reactions in Controlled Trials with an Incidence of 1% or Greater in Patients Administered ARTICADENT containing Epinephrine 1:100,000 Body System/Reaction ARTICADENT containing epinephrine
1:100,000 (N=882) IncidenceBody as a whole Face Edema 13 (1%) Headache 31 (4%) Infection 10 (1%) Pain 114 (13%) Digestive system Gingivitis 13 (1%) Nervous system Paresthesia 11 (1%) Table 3: Adverse Reactions in Controlled Trials with an Incidence of 1% or Greater in Patients Administered ARTICADENT containing Epinephrine 1:200,000 and ARTICADENT containing Epinephrine 1:100,000 Reaction ARTICADENT with epinephrine 1:200,000 (N=179) Incidence ARTICADENT with epinephrine 1:100,000 (N=182) Incidence Any adverse reaction 33 (18%) 35 (19%) Pain 11 (6.1%) 14 (7.6%) Headache 9 (5%) 6 (3.2%) Positive blood aspiration into syringe 3 (1.6%) 6 (3.2%) Swelling 3 (1.6%) 5 (2.7%) Trismus 1 (0.5%) 3 (1.6%) Nausea and emesis 3 (1.6%) 0 (0%) Sleepiness 2 (1.1%) 1 (0.5%) Numbness and tingling 1 (0.5%) 2 (1%) Palpitation 0 (0%) 2 (1%) Ear symptoms
(earache, otitis media)1 (0.5%) 2 (1%) Cough, persistent cough 0 (0%) 2 (1%) Adverse reactions observed in less than 1% of patients:
Table 4: Adverse Reactions in Controlled Trials with an Incidence of less than 1% but Considered Clinically Relevant in Patients Administered ARTICADENT Body System Reactions Body as a Whole Asthenia; back pain; injection site pain; burning sensation above injection site; malaise; neck pain Cardiovascular System Hemorrhage; migraine; syncope; tachycardia; elevated blood pressure Digestive System Dyspepsia; glossitis; gum hemorrhage; mouth ulceration; nausea; stomatitis; tongue edemas; tooth disorder; vomiting Hemic and Lymphatic System Ecchymosis; lymphadenopathy Metabolic and Nutritional System Edema; thirst Musculoskeletal System Arthralgia; myalgia; osteomyelitis Nervous System Dizziness; dry mouth; facial paralysis; hyperesthesia; increased salivation; nervousness; neuropathy; paresthesia; somnolence; exacerbation of Kearns-Sayre Syndrome Respiratory System Pharyngitis; rhinitis; sinus pain; sinus congestion Skin and Appendages Pruritus; skin disorder Special Senses Ear pain; taste perversion 6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ARTICADENT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Persistent paresthesias of the lips, tongue, and oral tissues have been reported with use of articaine hydrochloride, with slow, incomplete, or no recovery. These postmarketing events have been reported chiefly following nerve blocks in the mandible and have involved the trigeminal nerve and its branches.
Hypoesthesia has been reported with use of articaine, especially in pediatric age groups, which is usually reversible. Prolonged numbness can result in soft tissue injuries such as that of the lips and tongue in these age groups.
Ischemic injury and necrosis have been described following use of articaine with epinephrine and have been postulated to be due to vascular spasm of terminal arterial branches.
Paralysis of ocular muscles has been reported, especially after posterior, superior alveolar injections of articaine during dental anesthesia. Symptoms include diplopia, mydriasis, ptosis, and difficulty in abduction of the affected eye. These symptoms have been described as developing immediately after injection of the anesthetic solution and persisting one minute to several hours, with generally complete recovery.
-
7 DRUG INTERACTIONS
The administration of local anesthetic solutions containing epinephrine to patients receiving monoamine oxidase inhibitors, nonselective beta-adrenergic antagonists, or tricyclic antidepressants may produce severe, prolonged hypertension. Phenothiazines and butyrophenones may reduce or reverse the pressor effect of epinephrine. Concurrent use of these agents should be avoided; however, in situations when concurrent therapy is necessary, careful patient monitoring is essential [see Warnings and Precautions (5.2)].
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Table 5: Examples of Drugs Associated with Methemoglobinemia Class Examples Nitrates/Nitrites nitric oxide, nitroglycerin, nitroprusside, nitrous oxide Local anesthetics articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine Antineoplastic agents cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase Antibiotics dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides Antimalarials chloroquine, primaquine Anticonvulsants phenobarbital, phenytoin, sodium valproate Other drugs acetaminophen, metoclopramide, quinine, sulfasalazine -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects - Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women with ARTICADENT. Articaine hydrochloride and epinephrine (1:100,000) has been shown to increase fetal deaths and skeletal variations in rabbits when given in doses approximately 4 times the maximum recommended human dose (MRHD). ARTICADENT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In embryo-fetal toxicity studies in rabbits, 80 mg/kg, subcutaneously (approximately 4 times the MRHD based on body surface area) caused fetal death and increased fetal skeletal variations, but these effects may be attributable to severe maternal toxicity, including seizures, observed at this dose. In contrast, no embryo-fetal toxicities were observed when articaine and epinephrine (1:100,000) was administered subcutaneously throughout organogenesis at doses up to 40 mg/kg in rabbits and 80 mg/kg in rats (approximately 2 times the MRHD based on body surface area).
In pre- and postnatal developmental studies subcutaneous administration of articaine hydrochloride to pregnant rats throughout gestation and lactation, at a dose of 80 mg/kg (approximately 2 times the MRHD based on body surface area) increased the number of stillbirths and adversely affected passive avoidance, a measure of learning, in pups. This dose also produced severe maternal toxicity in some animals. A dose of 40 mg/kg (approximately equal to the MRHD on a mg/m2 basis) did not produce these effects. A similar study using articaine and epinephrine (1:100,000) rather than articaine hydrochloride alone produced maternal toxicity, but no effects on offspring.
8.3 Nursing Mothers
It is not known whether ARTICADENT is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ARTICADENT is administered to a nursing woman. When using ARTICADENT, nursing mothers may choose to pump and discard breast milk for approximately 4 hours (based on plasma half-life) following an injection of ARTICADENT (to minimize infant ingestion) and then resume breastfeeding.
8.4 Pediatric Use
Safety and effectiveness of ARTICADENT in pediatric patients below the age of 4 years have not been established. Safety of doses greater than 7 mg/kg (0.175 mL/kg) in pediatric patients has not been established.
The safety and effectiveness of ARTICADENT for local, infiltrative, or conductive anesthesia in both simple and complex dental procedures have been established in pediatric patients ages 4 to 16 years old. Safety and effectiveness was established in clinical trials with 61 pediatric patients between the ages of 4 and 16 years administered articaine hydrochloride 4% and epinephrine 1:100,000 injections. Fifty-one of these patients received doses from 0.76 mg/kg to 5.65 mg/kg (0.9 to 5.1 mL) for simple dental procedures and 10 patients received doses between 0.37 mg/kg and 7.48 mg/kg (0.7 to 3.9 mL) for complex dental procedures. Approximately 13% of these pediatric patients required additional injections of anesthetic for complete anesthesia. Dosages in pediatric patients should be reduced, commensurate with age, body weight, and physical condition [see Dosage and Administration (2.2)].
8.5 Geriatric Use
In clinical trials, 54 patients between the ages of 65 and 75 years, and 11 patients 75 years and over received ARTICADENT containing epinephrine 1:100,000. Among all patients between 65 and 75 years, doses from 0.43 mg/kg to 4.76 mg/kg (0.9 to 11.9 mL) were administered to 35 patients for simple procedures and doses from 1.05 mg/kg to 4.27 mg/kg (1.3 to 6.8 mL) were administered to 19 patients for complex procedures. Among the 11 patients ≥ 75 years old, doses from 0.78 mg/kg to 4.76 mg/kg (1.3 to 11.9 mL) were administered to 7 patients for simple procedures and doses of 1.12 mg/kg to 2.17 mg/kg (1.3 to 5.1 mL) were administered to 4 patients for complex procedures. Approximately 6% of patients between the ages of 65 and 75 years and none of the 11 patients 75 years of age or older required additional injections of anesthetic for complete anesthesia compared with 11% of patients between 17 and 65 years old who required additional injections.
No overall differences in safety or effectiveness were observed between elderly subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal and Hepatic Impairment
No studies have been performed with articaine hydrochloride 4% and epinephrine 1:200,000 injection or articaine hydrochloride 4% and epinephrine 1:100,000 injection in patients with renal or hepatic impairment [see Warnings and Precautions (5.2)].
-
10 OVERDOSAGE
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local anesthetic solution [see Warnings and Precautions (5.1, 5.2)].
The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient's state of consciousness after each local anesthetic injection. At the first sign of change, oxygen should be administered.
The first step in the management of convulsions, as well as hypo-ventilation, consists of immediate attention to the maintenance of a patent airway and assisted or controlled ventilation as needed. The adequacy of the circulation should be assessed. Should convulsions persist despite adequate respiratory support, treatment with appropriate anticonvulsant therapy is indicated. The practitioner should be familiar with the use of anticonvulsant drugs, prior to the use of local anesthetics. Supportive treatment of circulatory depression may require administration of intravenous fluids and, when appropriate, a vasopressor.
If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias, and/or cardiac arrest. If cardiac arrest should occur, standard cardiopulmonary resuscitative measures should be instituted.
For additional information about overdose treatment, call a poison control center (1-800-222-1222).
-
11 DESCRIPTION
ARTICADENT (articaine hydrochloride and epinephrine injection), for intraoral submucosal infiltration use, is a sterile, aqueous solution that contains articaine HCl 4% (40 mg/mL) and epinephrine bitartrate in an epinephrine 1:200,000 or epinephrine 1:100,000 strength.
Articaine HCl is an amino amide local anesthetic, chemically designated as 4-methyl-3-[2-(propylamino)-propionamido]-2-thiophene-carboxylic acid, methyl ester hydrochloride and is a racemic mixture. Articaine HCl has a molecular weight of 320.84 and the following structural formula:
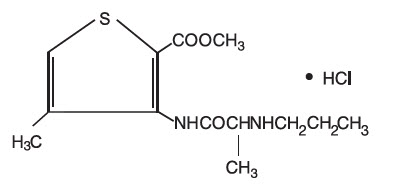
Articaine HCl has a partition coefficient in n-octanol/Soerensen buffer (pH 7.35) of 17 and a pKa of 7.8.
Epinephrine bitartrate, (-)-1-(3,4-Dihydroxyphenyl)-2-methylamino-ethanol (+) tartrate (1:1) salt, is a vasoconstrictor with a concentration of 1:200,000 or 1:100,000 (expressed as free base). It has a molecular weight of 333.3 and the following structural formula:
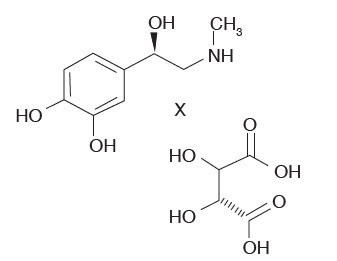
ARTICADENT contains the following inactive ingredients: sodium chloride (1.6 mg/mL), and sodium metabisulfite (0.5 mg/mL). The product is formulated with a 15% overage of epinephrine. The pH is adjusted with sodium hydroxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Articaine HCl is an amide local anesthetic. Local anesthetics block the generation and conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination, and conduction velocity of the affected nerve fibers. Epinephrine is a vasoconstrictor added to articaine HCl to slow absorption into the general circulation and thus prolong maintenance of an active tissue concentration.
12.2 Pharmacodynamics
Clinically, the order of loss of nerve function is as follows: (1) pain; (2) temperature; (3) touch; (4) proprioception; and (5) skeletal muscle tone.
The onset of anesthesia has been shown to be within 1 to 9 minutes of injection of ARTICADENT. Complete anesthesia lasts approximately 1 hour for infiltrations and up to approximately 2 hours for nerve block.
Administration of ARTICADENT results in a 3- to 5-fold increase in plasma epinephrine concentrations compared to baseline; however, in healthy adults it does not appear to be associated with marked increases in blood pressure or heart rate, except in the case of accidental intravascular injection [see Warnings and Precautions (5.1)].
12.3 Pharmacokinetics
Absorption:
Following dental injection by the submucosal route of an articaine solution containing epinephrine 1:200,000, articaine reaches peak blood concentration about 25 minutes after a single dose injection and 48 minutes after three doses. Peak plasma levels of articaine achieved after 68 and 204 mg doses are 385 and 900 ng/mL, respectively.
Following intraoral administration of a near maximum dose of 476 mg, articaine reaches peak blood concentrations of 2037 and 2145 ng/mL for articaine solution containing epinephrine 1:100,000 and 1:200,000, respectively, approximately 22 minutes post-dose.
Distribution:
Approximately 60 to 80% of articaine HCl is bound to human serum albumin and y-globulins at 37°C in vitro.
Elimination:
Metabolism: Articaine HCl is metabolized by plasma carboxyesterase to its primary metabolite, articainic acid, which is inactive. In vitro studies show that the human liver microsome P450 isoenzyme system metabolizes approximately 5% to 10% of available articaine with nearly quantitative conversion to articainic acid.
Excretion: At the dose of 476 mg of articaine, the elimination half-life was 43.8 minutes and 44.4 minutes for articaine solution containing epinephrine 1:100,000 and 1:200,000, respectively. Articaine is excreted primarily through urine with 53-57% of the administered dose eliminated in the first 24 hours following submucosal administration. Articainic acid is the primary metabolite in urine. A minor metabolite, articainic acid glucuronide, is also excreted in urine. Articaine constitutes only 2% of the total dose excreted in urine.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to evaluate the carcinogenic potential of articaine HCl in animals have not been conducted. Five standard mutagenicity tests, including three in vitro tests (the nonmammalian Ames test, the mammalian Chinese hamster ovary chromosomal aberration test, and a mammalian gene mutation test with articaine HCl) and two in vivo mouse micronucleus tests (one with articaine and epinephrine 1:100,000 and one with articaine HCl alone) showed no mutagenic effects.
No effects on male or female fertility were observed in rats for articaine and epinephrine 1:100,000 administered subcutaneously in doses up to 80 mg/kg/day (approximately 2 times the MRHD based on body surface area).
-
14 CLINICAL STUDIES
Three randomized, double-blind, active-controlled studies were designed to evaluate effectiveness of ARTICADENT containing epinephrine 1:100,000 as a dental anesthetic. Patients ranging in age from 4 years to over 65 years old underwent simple dental procedures such as single uncomplicated extractions, routine operative procedures, single apical resections, and single crown procedures, or complex dental procedures such as multiple extractions, multiple crowns and/or bridge procedures, multiple apical resections, alveolectomies, mucogingival operations, and other surgical procedures on the bone. ARTICADENT containing epinephrine 1:100,000 was administered by intraoral submucosal infiltration and/or nerve block for these dental procedures.
Efficacy was measured immediately following the procedure by having the patient and investigator rate the patient's procedural pain using a 10 cm visual analog scale (VAS), in which a score of zero represented no pain and a score of 10 represented the worst pain imaginable. Mean patient and investigator VAS pain scores were 0.3-0.4 cm for simple procedures and 0.5-0.6 cm for complex procedures.
Four randomized, double-blind, active-controlled studies were performed comparing ARTICADENT containing epinephrine 1:100,000 versus ARTICADENT containing epinephrine 1:200,000. The first two studies used electric pulp testers (EPT) to evaluate the success rate (maximum EPT value within 10 minutes), onset, and duration of ARTICADENT containing epinephrine 1:100,000 versus ARTICADENT containing epinephrine 1:200,000 and articaine solution without epinephrine in healthy adults between 18 and 65 years old. Results indicated that the anesthetic characteristics of the 1:100,000 and 1:200,000 formulations are not significantly different.
A third study compared the difference in visualization of the surgical field after administration of ARTICADENT containing epinephrine 1:100,000 versus ARTICADENT containing epinephrine 1:200,000 during bilateral maxillary periodontal surgeries in patients ranging from 21 to 65 years old. ARTICADENT containing epinephrine 1:100,000 provided better visualization of the surgical field and less blood loss during the procedures.
In a fourth study, designed to assess and compare cardiovascular safety, when the maximum dose of each formulation was administered, no clinically relevant differences in blood pressure or heart rate between formulations were observed.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
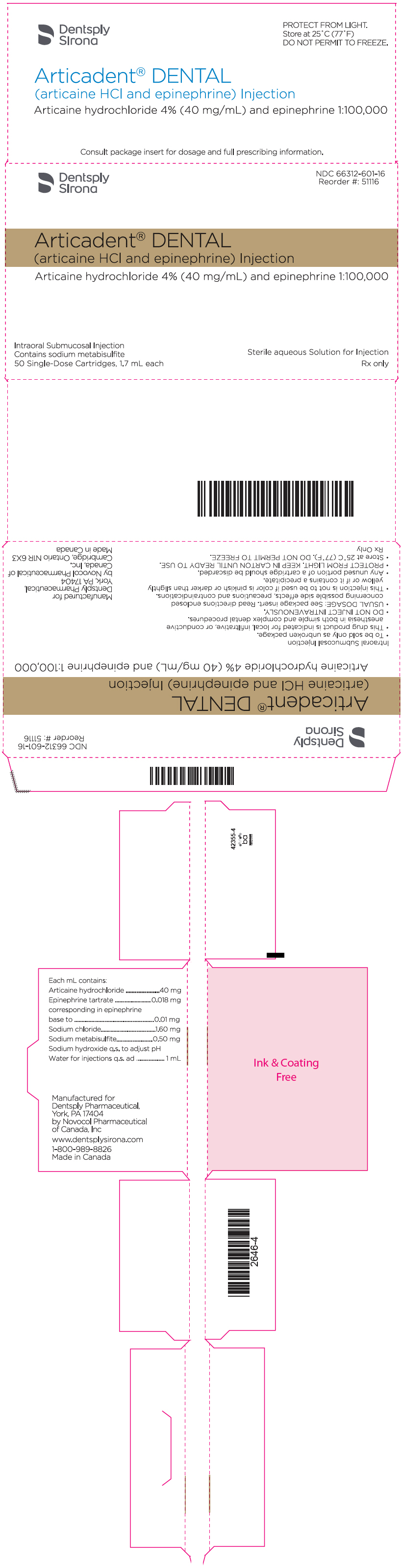
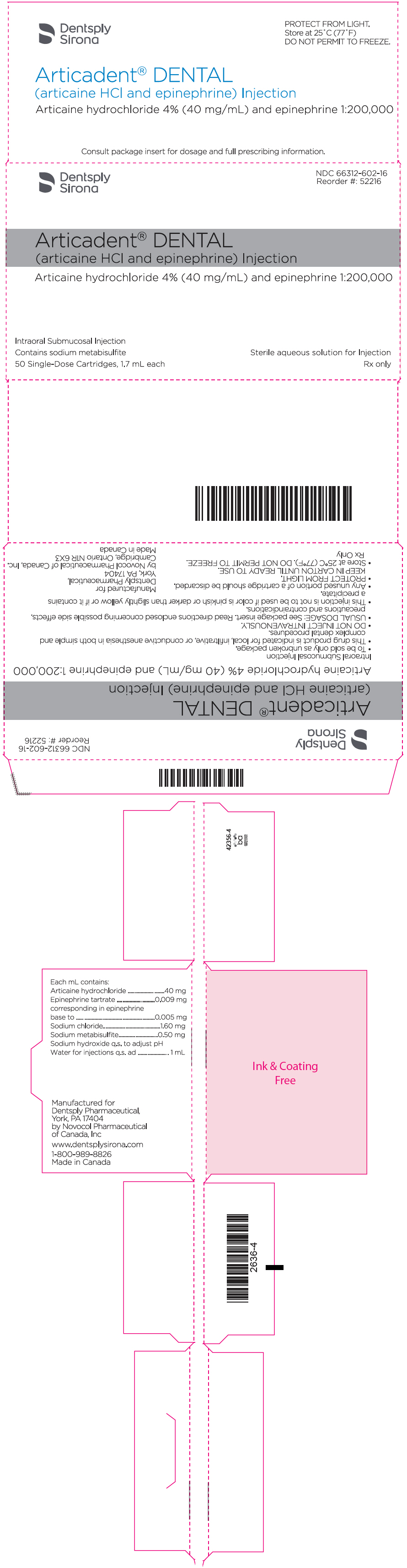
ARTICADENT (articaine hydrochloride and epinephrine) injection is a clear, colorless solution available in 1.7 mL single-dose glass cartridges, packaged in boxes of 50 cartridges in the following two strengths (less than a full cartridge or more than one cartridge may be used for an individual patient):
- ARTICADENT containing articaine HCl 4% (40 mg/mL) and epinephrine 1:200,000 (as epinephrine bitartrate 0.009 mg/mL) (NDC 66312-602-16)
- ARTICADENT containing articaine HCl 4% (40 mg/mL) and epinephrine 1:100,000 (as epinephrine bitartrate 0.018 mg/mL) (NDC 66312-601-16)
-
17 PATIENT COUNSELING INFORMATION
Loss of Sensation and Muscle Function:
Inform patients in advance of the possibility of temporary loss of sensation and muscle function following infiltration and nerve block injections [see Adverse Reactions (6.2)].
Instruct patients not to eat or drink until normal sensation returns.
Methemoglobinemia:
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 1.7 mL Cartridge Carton - epinephrine 1:100,000
Dentsply
SironaNDC 66312-601-16
Reorder #: 51116Articadent® DENTAL
(articaine HCl and epinephrine) InjectionArticaine hydrochloride 4% (40 mg/mL) and epinephrine 1:100,000
Intraoral Submucosal Injection
Contains sodium metabisulfite
50 Single-Dose Cartridges, 1.7 mL eachSterile aqueous Solution for Injection
Rx only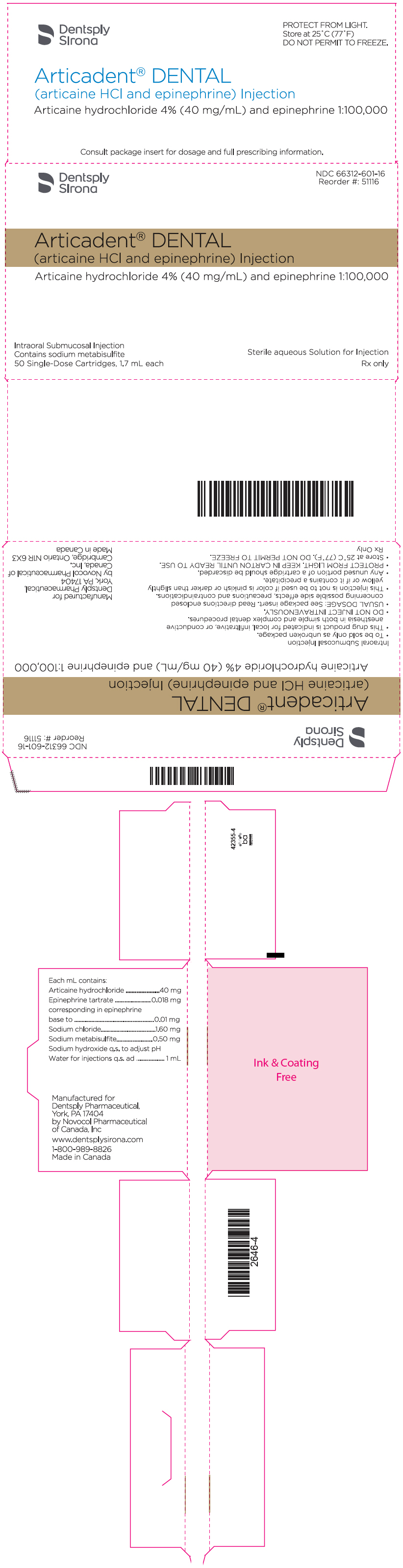
-
PRINCIPAL DISPLAY PANEL - 1.7 mL Cartridge Carton - epinephrine 1:200,000
Dentsply
SironaNDC 66312-602-16
Reorder #: 52216Articadent® DENTAL
(articaine HCl and epinephrine) InjectionArticaine hydrochloride 4% (40 mg/mL) and epinephrine 1:200,000
Intraoral Submucosal Injection
Contains sodium metabisulfite
50 Single-Dose Cartridges, 1.7 mL eachSterile aqueous solution for Injection
Rx only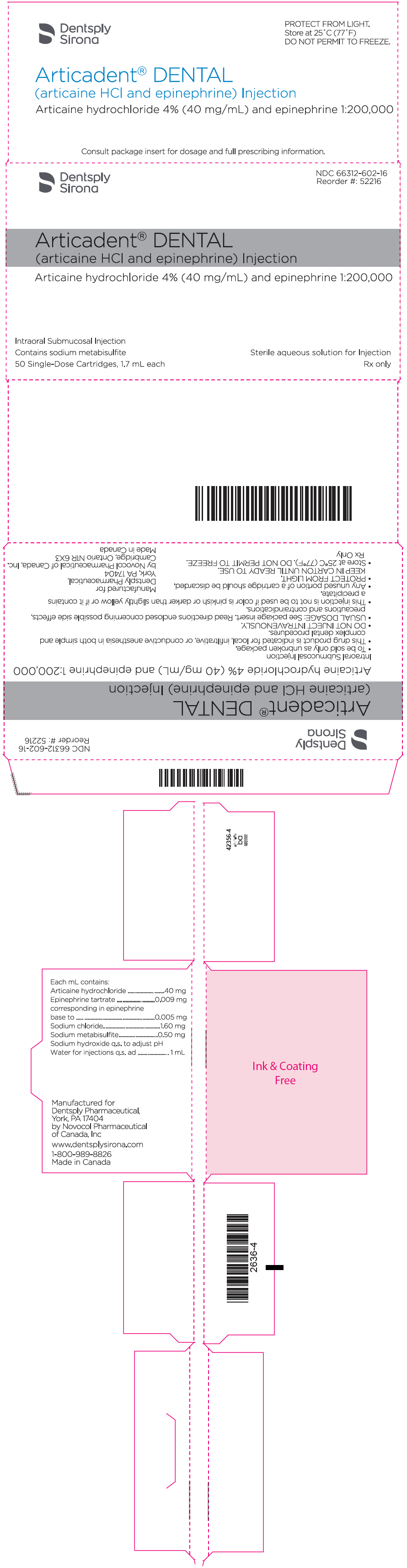
-
INGREDIENTS AND APPEARANCE
ARTICADENT
articaine hydrochloride and epinephrine bitartrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66312-601 Route of Administration SUBMUCOSAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARTICAINE HYDROCHLORIDE (UNII: QS9014Q792) (ARTICAINE - UNII:D3SQ406G9X) ARTICAINE HYDROCHLORIDE 40 mg in 1 mL EPINEPHRINE BITARTRATE (UNII: 30Q7KI53AK) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.01 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 1.6 mg in 1 mL SODIUM METABISULFITE (UNII: 4VON5FNS3C) 0.5 mg in 1 mL WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66312-601-16 50 in 1 CARTON 09/01/2009 1 1.7 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA020971 09/01/2009 ARTICADENT
articaine hydrochloride and epinephrine bitartrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66312-602 Route of Administration SUBMUCOSAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARTICAINE HYDROCHLORIDE (UNII: QS9014Q792) (ARTICAINE - UNII:D3SQ406G9X) ARTICAINE HYDROCHLORIDE 40 mg in 1 mL EPINEPHRINE BITARTRATE (UNII: 30Q7KI53AK) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 0.005 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 1.6 mg in 1 mL SODIUM METABISULFITE (UNII: 4VON5FNS3C) 0.5 mg in 1 mL WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66312-602-16 50 in 1 CARTON 09/01/2009 1 1.7 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA020971 09/01/2009 Labeler - Dentsply Pharmaceutical (102221942) Establishment Name Address ID/FEI Business Operations Novocol Pharmaceutical of Canada, Inc. 201719960 manufacture(66312-601, 66312-602)