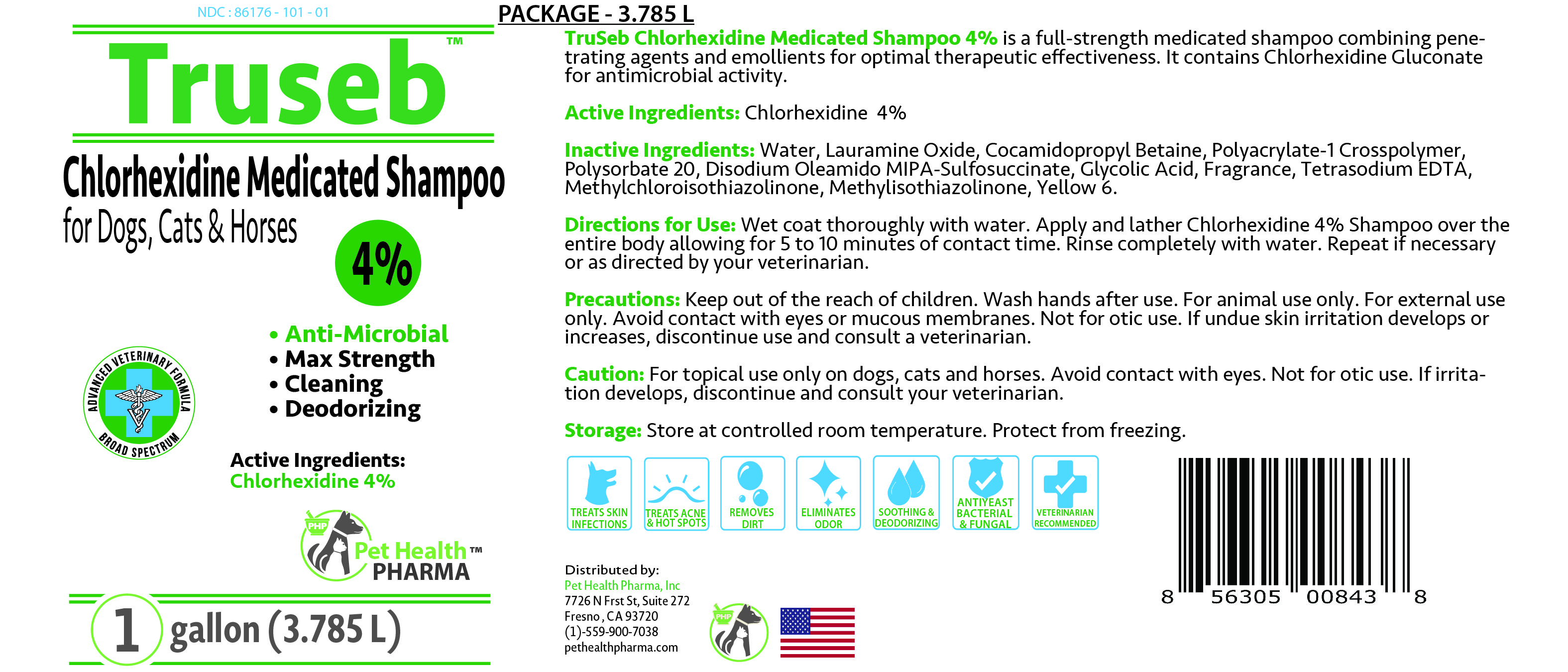
Label: TRUSEB CHLORHEXIDINE 4% MEDICATED FOR DOGS, CATS AND HORSES- chlorhexidine gluconate shampoo
- NDC Code(s): 86176-101-01, 86176-101-08, 86176-101-12, 86176-101-16
- Packager: Pet Health Pharma, LLC
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients:
- PURPOSE:
- INACTIVE INGREDIENT:
- INDICATIONS & USAGE:
- DOSAGE & ADMINISTRATION:
- PRECAUTIONS:
- CAUTION:
- STORAGE:
-
SPL UNCLASSIFIED SECTION:
Chlorhexidine Medicated Shampoo
for Dogs, Cats & Horses
Chlorhexidine 4%
• Anti-Microbial
• Max Strength
• Cleaning
• DeodorizingADVANCED VETERINARY FORMULA
BROAD SPECTRUM
TREATS SKIN INFECTIONS
TREATS ACNE & HOT SPOTS
REMOVES DIRT
ELIMINATES ORDER
ANTIYEAST BACTERIAL & FUNGAL
SOOTHING & DEODORIZING
VETERINARIAN RECOMMENDED
Distributed by:
Pet Health Pharma, Inc
7726 N Frst St, Suite 272
Fresno , CA 93720
(1)-559-900-7038
pethealthpharma.comMADE IN USA
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRUSEB CHLORHEXIDINE 4% MEDICATED FOR DOGS, CATS AND HORSES
chlorhexidine gluconate shampooProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86176-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) POLYACRYLATE-1 CROSSPOLYMER (UNII: R5P9Z5WD6D) POLYSORBATE 20 (UNII: 7T1F30V5YH) DISODIUM OLEAMIDO MIPA-SULFOSUCCINATE (UNII: 0MBZ20845F) GLYCOLIC ACID (UNII: 0WT12SX38S) EDETATE SODIUM (UNII: MP1J8420LU) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86176-101-08 237 mL in 1 BOTTLE 2 NDC:86176-101-12 355 mL in 1 BOTTLE 3 NDC:86176-101-16 473 mL in 1 BOTTLE 4 NDC:86176-101-01 3785 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2021 Labeler - Pet Health Pharma, LLC (100789128)