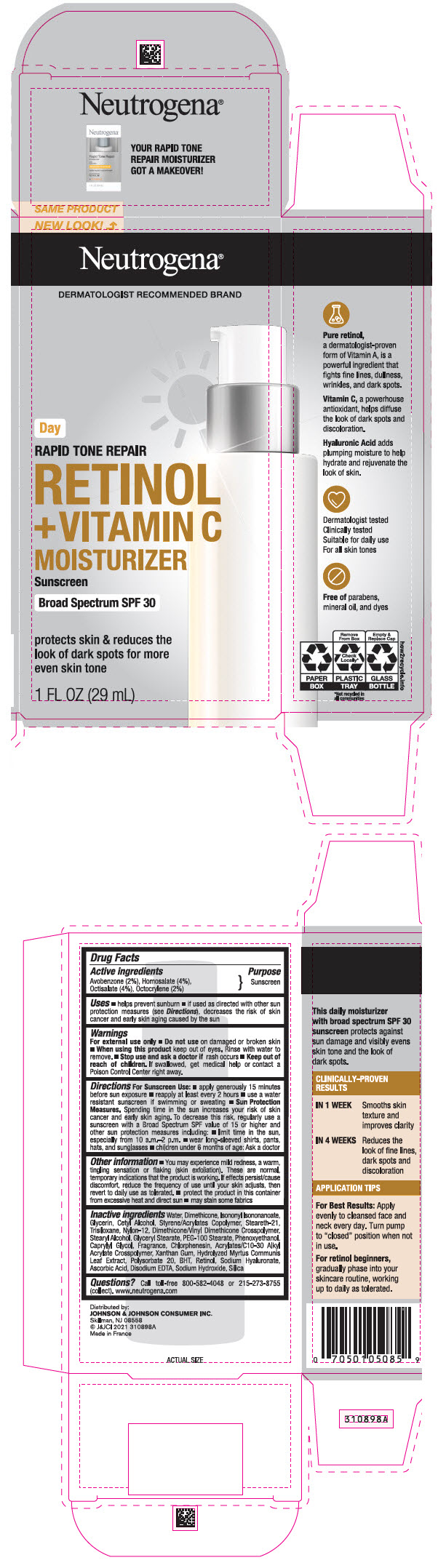
Label: NEUTROGENA RAPID TONE REPAIR RETINOL PLUS VITAMIN C MOISTURIZER SUNSCREEN BROAD SPECTRUM SPF 30 DAY- avobenzone, homosalate, octisalate, and octocrylene lotion
- NDC Code(s): 69968-0705-1
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For Sunscreen Use:
- apply generously 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
-
Other information
- You may experience mild redness, a warm, tingling sensation or flaking (skin exfoliation). These are normal, temporary indications that the formula is working. If effects persist/cause discomfort, reduce the frequency of use until your skin adjusts, then revert to daily use as tolerated.
- protect this product from excessive heat and direct sun
- may stain some fabrics
-
Inactive ingredients
Water, Dimethicone, Isononyl Isononanoate, Glycerin, Cetyl Alcohol, Styrene/Acrylates Copolymer, Steareth-21, Trisiloxane, Nylon-12, Dimethicone/Vinyl Dimethicone Crosspolymer, Stearyl Alcohol, Glyceryl Stearate, PEG-100 Stearate, Phenoxyethanol, Caprylyl Glycol, Fragrance, Chlorphenesin, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Xanthan Gum, Hydrolyzed Myrtus Communis Leaf Extract, Polysorbate 20, BHT, Retinol, Sodium Hyaluronate, Ascorbic Acid, Disodium EDTA, Sodium Hydroxide, Silica
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 29 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
NEUTROGENA RAPID TONE REPAIR RETINOL PLUS VITAMIN C MOISTURIZER SUNSCREEN BROAD SPECTRUM SPF 30 DAY
avobenzone, homosalate, octisalate, and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0705 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 20 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 40 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 40 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) STEARETH-21 (UNII: 53J3F32P58) TRISILOXANE (UNII: 9G1ZW13R0G) NYLON-12 (UNII: 446U8J075B) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) XANTHAN GUM (UNII: TTV12P4NEE) POLYSORBATE 20 (UNII: 7T1F30V5YH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) RETINOL (UNII: G2SH0XKK91) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ASCORBIC ACID (UNII: PQ6CK8PD0R) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0705-1 1 in 1 CARTON 06/01/2021 08/29/2024 1 29 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/01/2021 08/29/2024 Labeler - Johnson & Johnson Consumer Inc. (118772437)