Label: HYDROCORTISONE cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 76413-339-28 - Packager: Central Texas Community Health Centers
- This is a repackaged label.
- Source NDC Code(s): 45802-438
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 28, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
-
Uses
- •
- temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
- •
- eczema
- •
- psoriasis
- •
- poison ivy, oak, sumac
- •
- insect bites
- •
- detergents
- •
- jewelry
- •
- seborrheic dermatitis
- •
- soaps
- •
- external genital and anal itching
- •
- other uses of this product should be only under the advice and supervision of a doctor
-
Warnings
For external use only
Do not use
- •
- for the treatment of diaper rash. Ask a doctor.
- •
- for external genital itching if you have a vaginal discharge. Ask a doctor.
When using this product
- •
- avoid contact with the eyes
- •
- do not begin use of any other hydrocortisone product unless you have asked a doctor
- •
- for external anal itching do not exceed the recommended daily dosage unless directed by a doctor. In case of bleeding, consult a doctor promptly.
- •
- do not put this product into the rectum by using fingers or any mechanical device or applicator
-
Directions
adults and children 2 years of age and older:
- •
- apply to affected area not more than 3 to 4 times daily
children under 2 years of age:
- •
- do not use. Ask a doctor.
for external anal itching:
- •
- adults: when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
- •
- children under 12 years of age with external anal itching: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
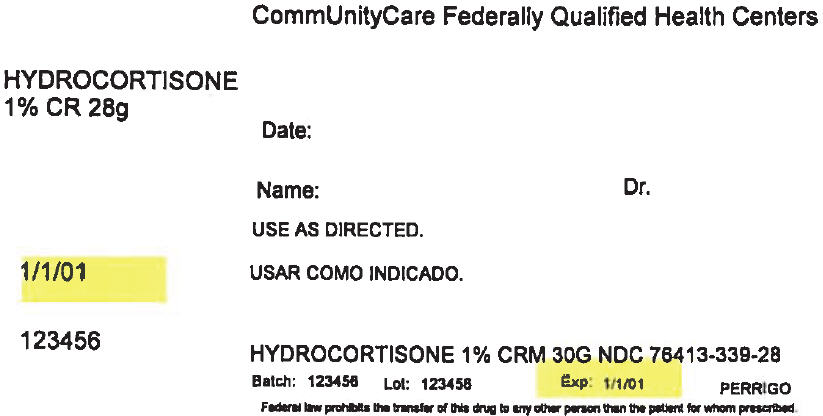
PRINCIPAL DISPLAY PANEL - 28 g Tube Label
CommUnityCare Federally Qualified Health Centers
HYDROCORTISONE
1% CR 28gDate:
Name:
Dr.USE AS DIRECTED.
1/1/01
123456
HYDROCORTISONE 1% CRM 30G NDC 76413-339-28
Batch: 123456
Lot: 123456
Exp: 1/1/01
PERRIGOFederal law prohibits the transfer of this drug to any other person than the patient for whom prescribed.
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76413-339(NDC:45802-438) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL PALMITATE (UNII: 5ZA2S6B08X) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76413-339-28 28 g in 1 TUBE; Type 0: Not a Combination Product 04/13/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 04/13/2011 Labeler - Central Texas Community Health Centers (079674019) Establishment Name Address ID/FEI Business Operations Central Texas Community Health Centers 079674019 REPACK(76413-339) , RELABEL(76413-339)

