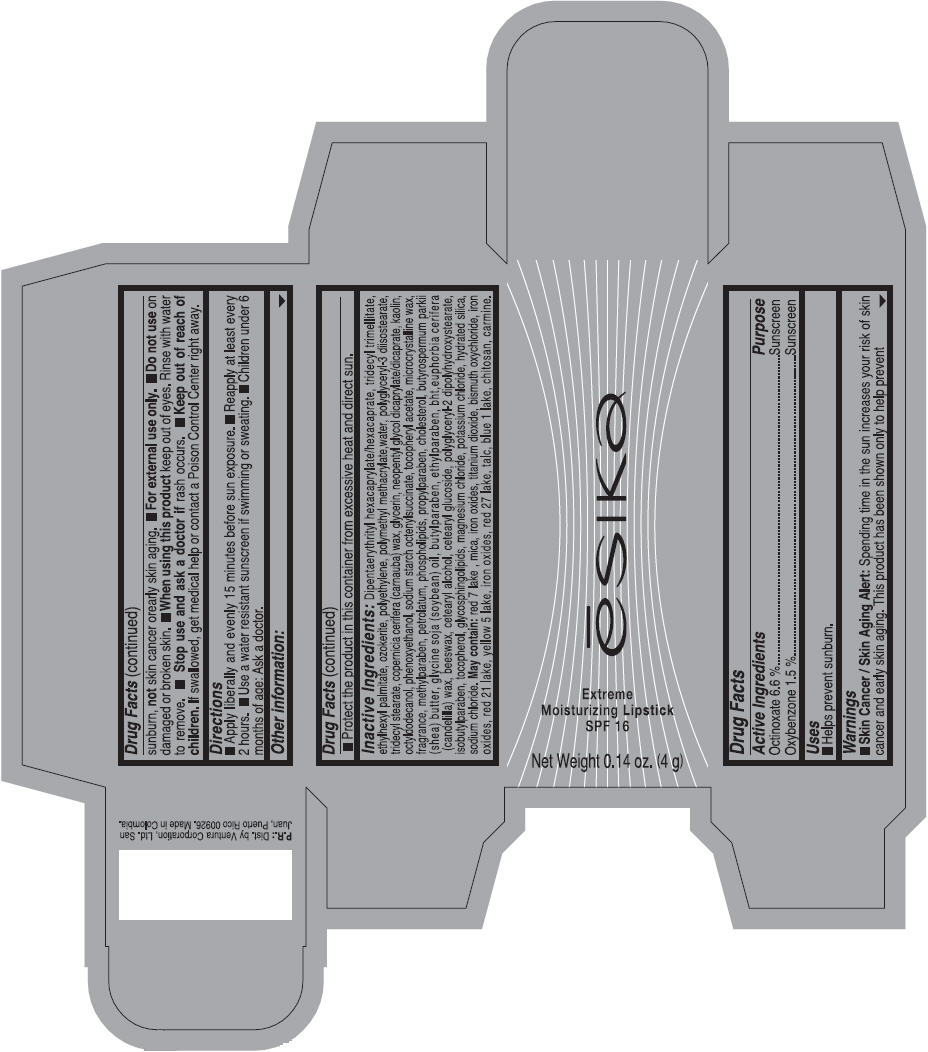
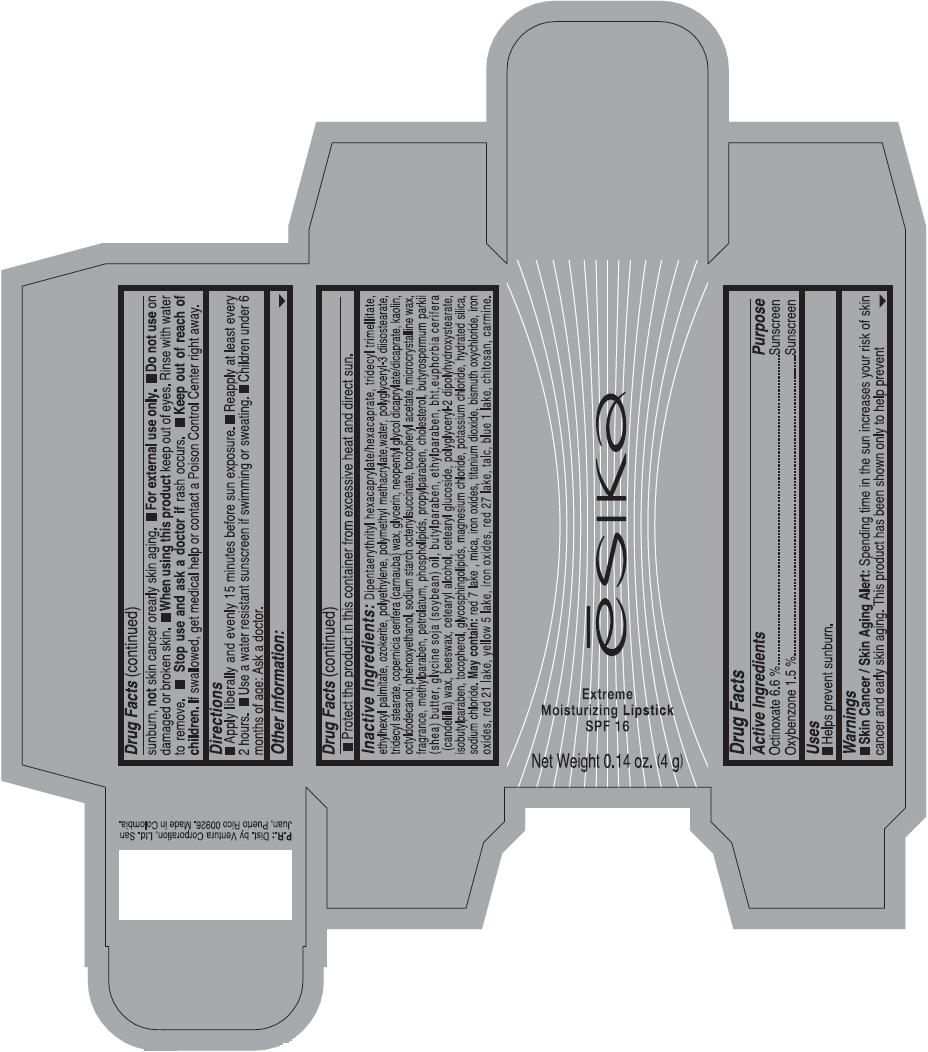
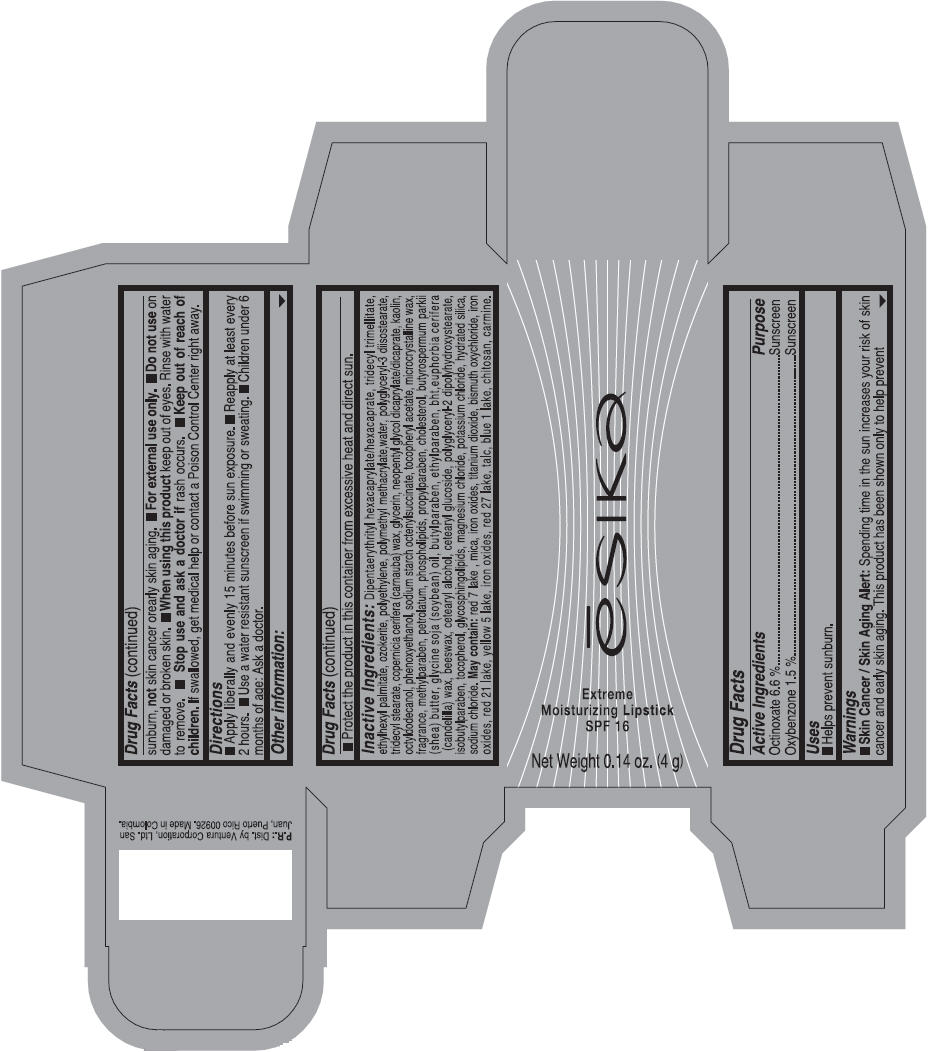
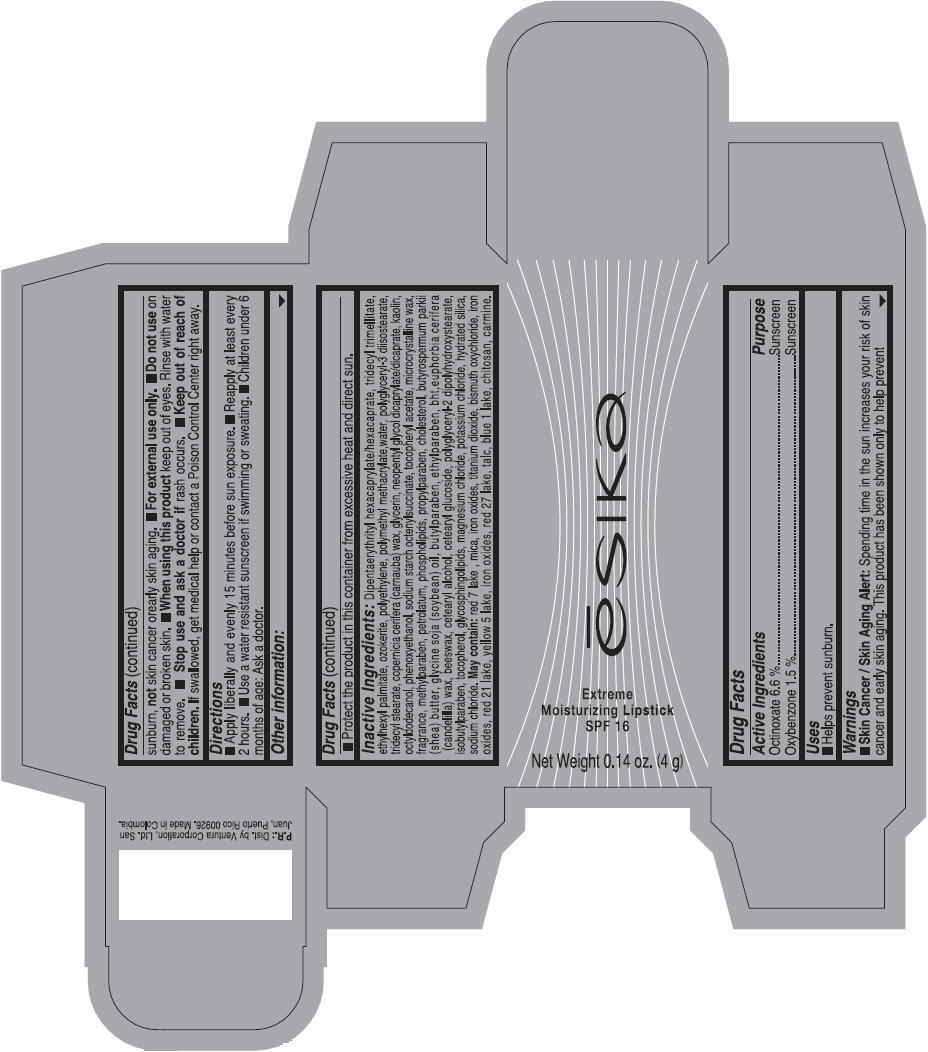
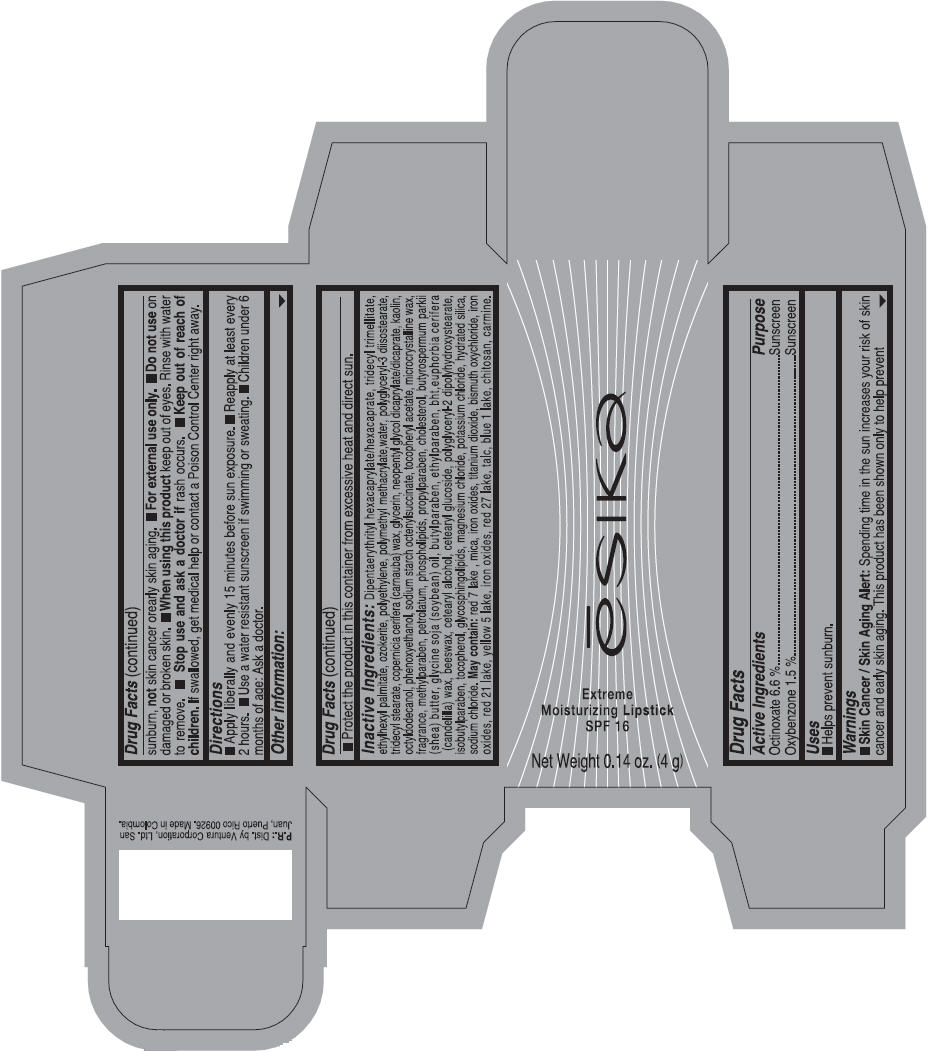
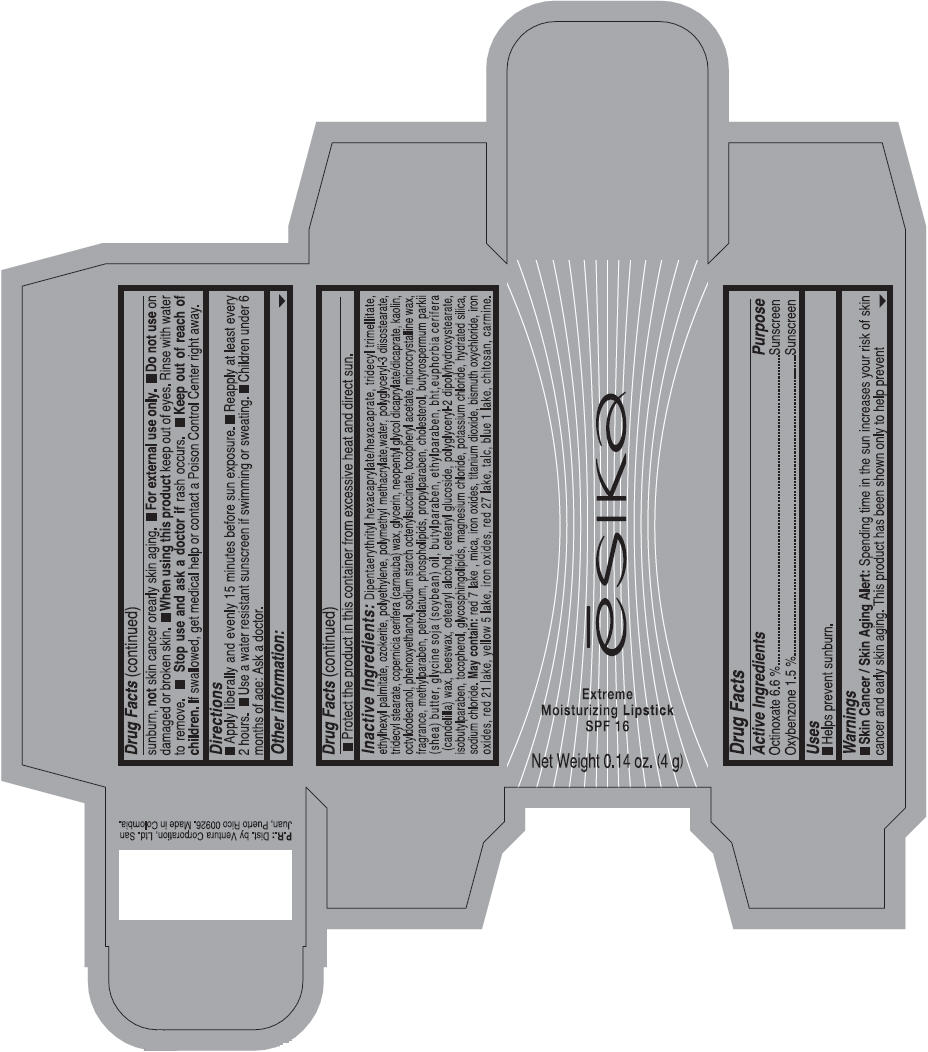
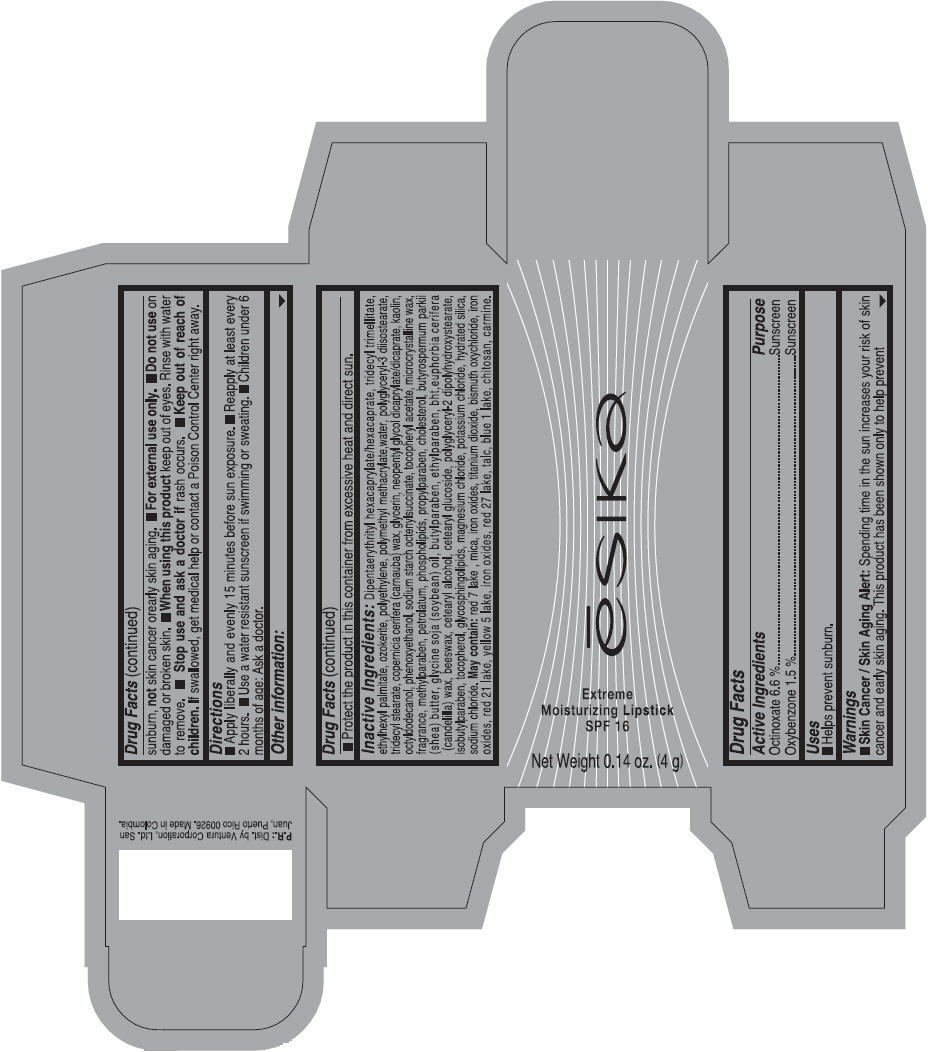
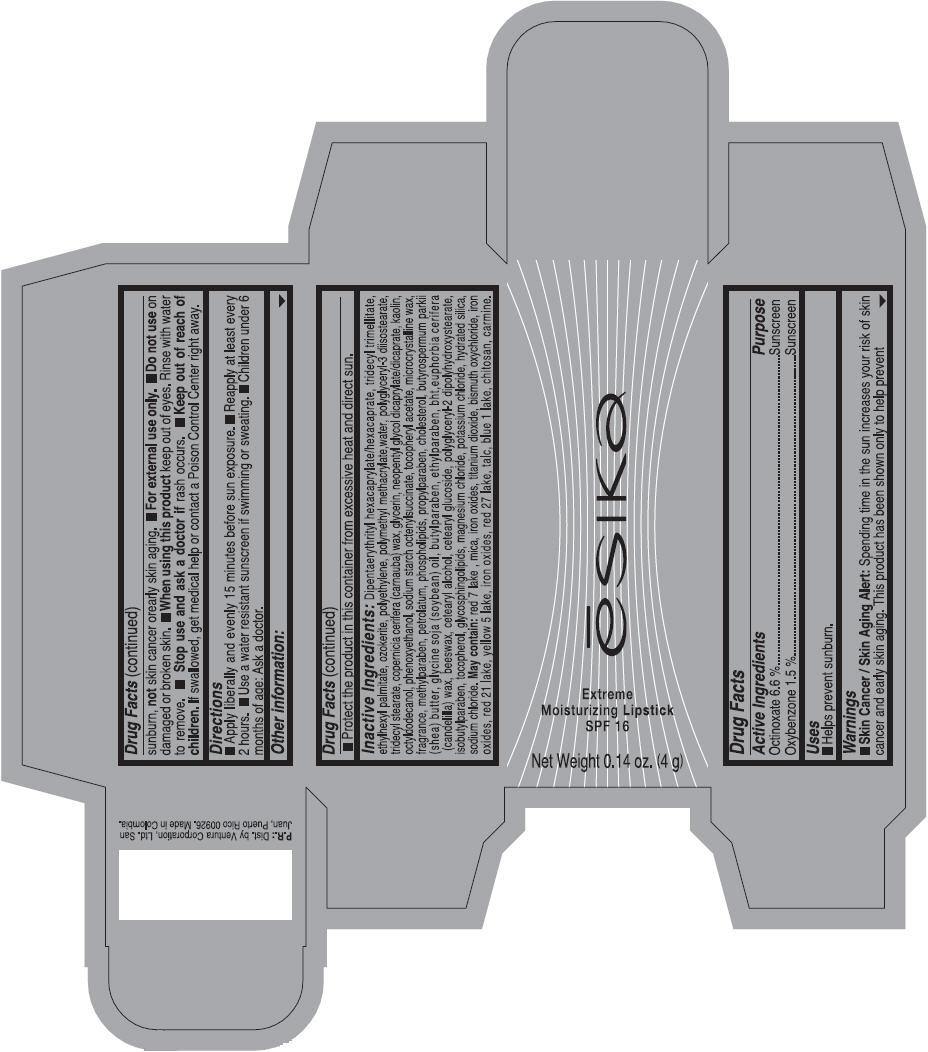
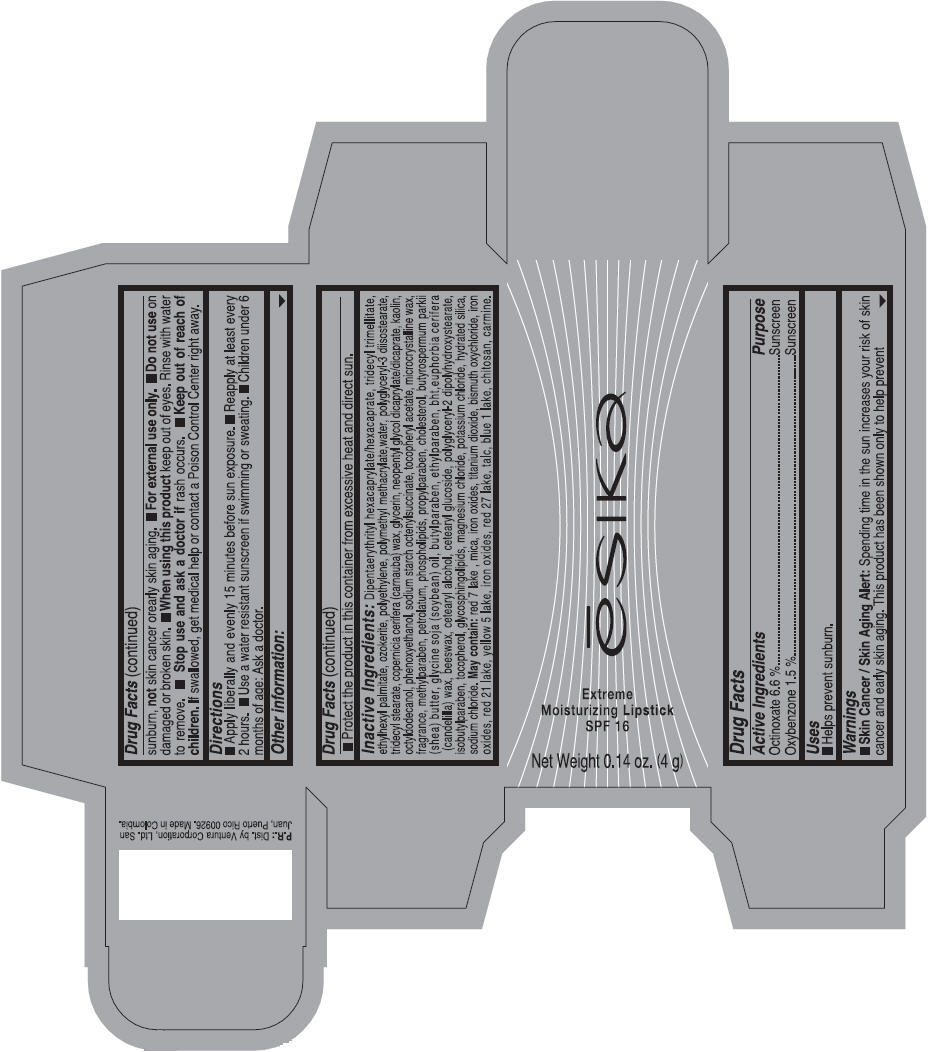
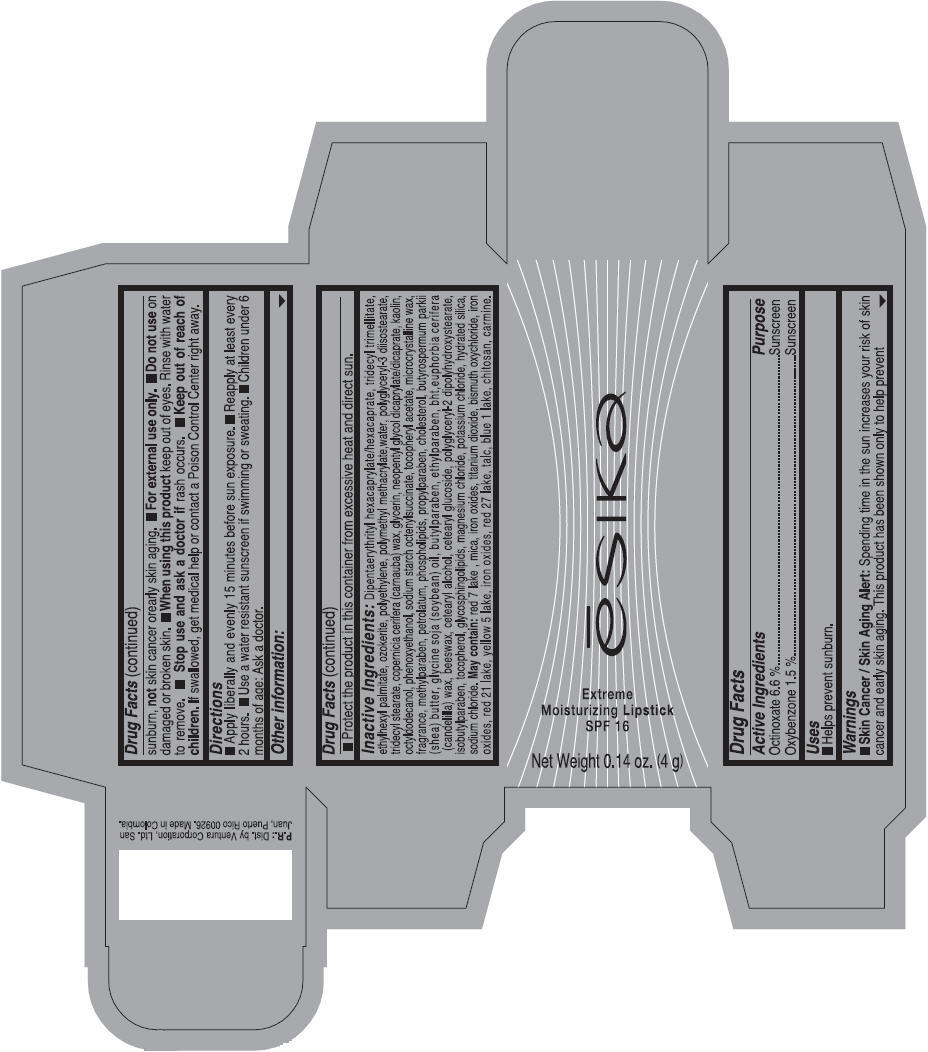
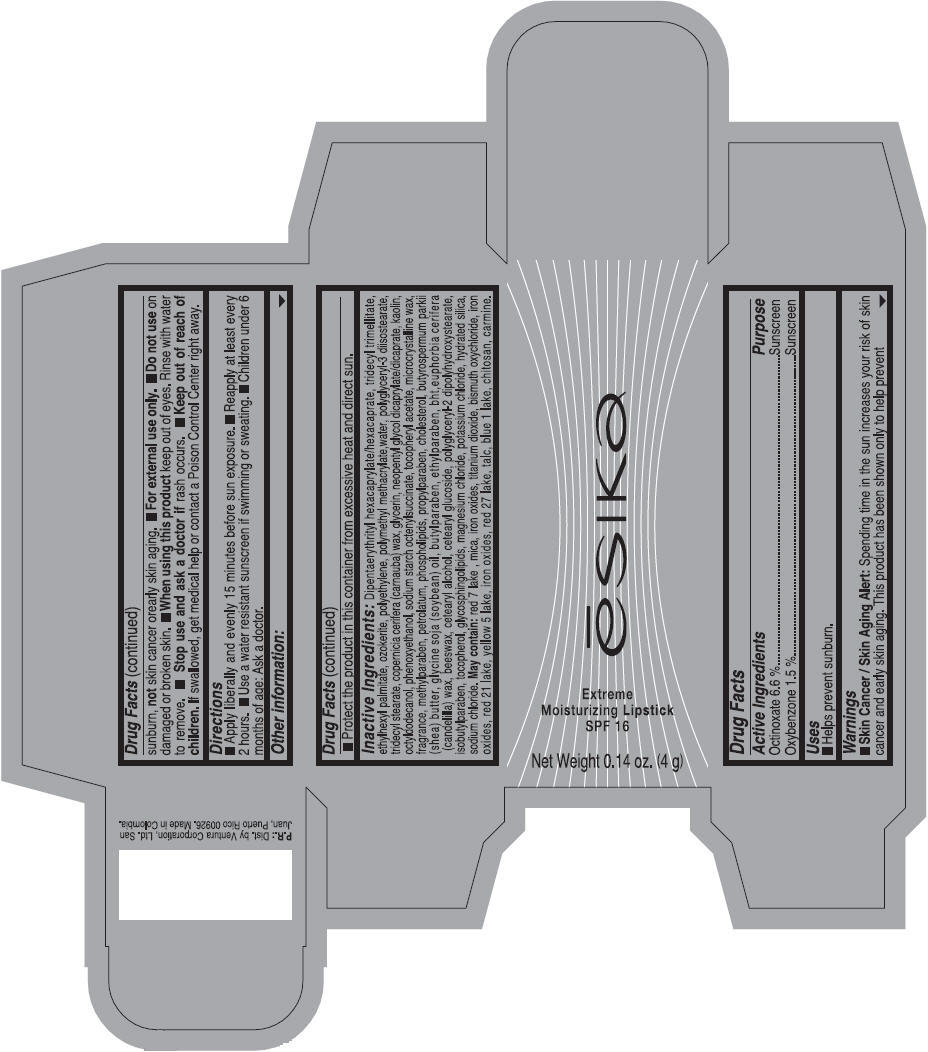
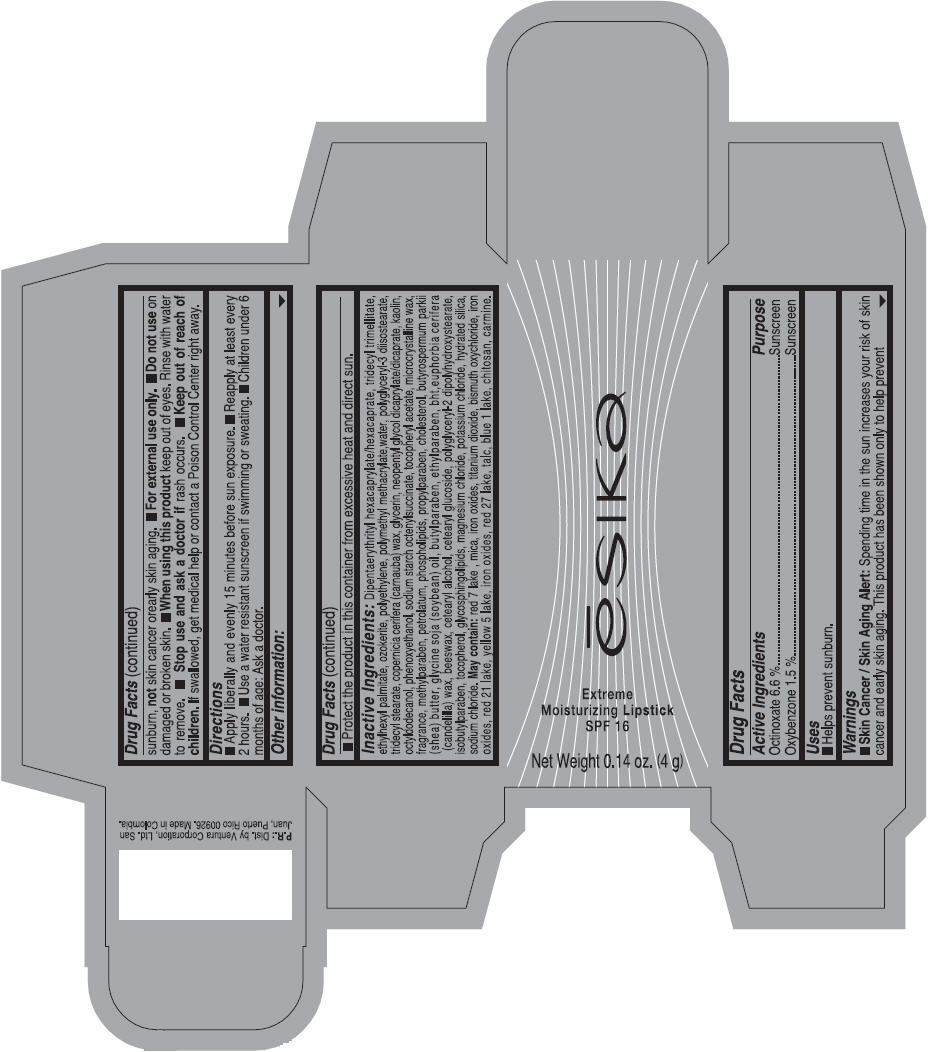
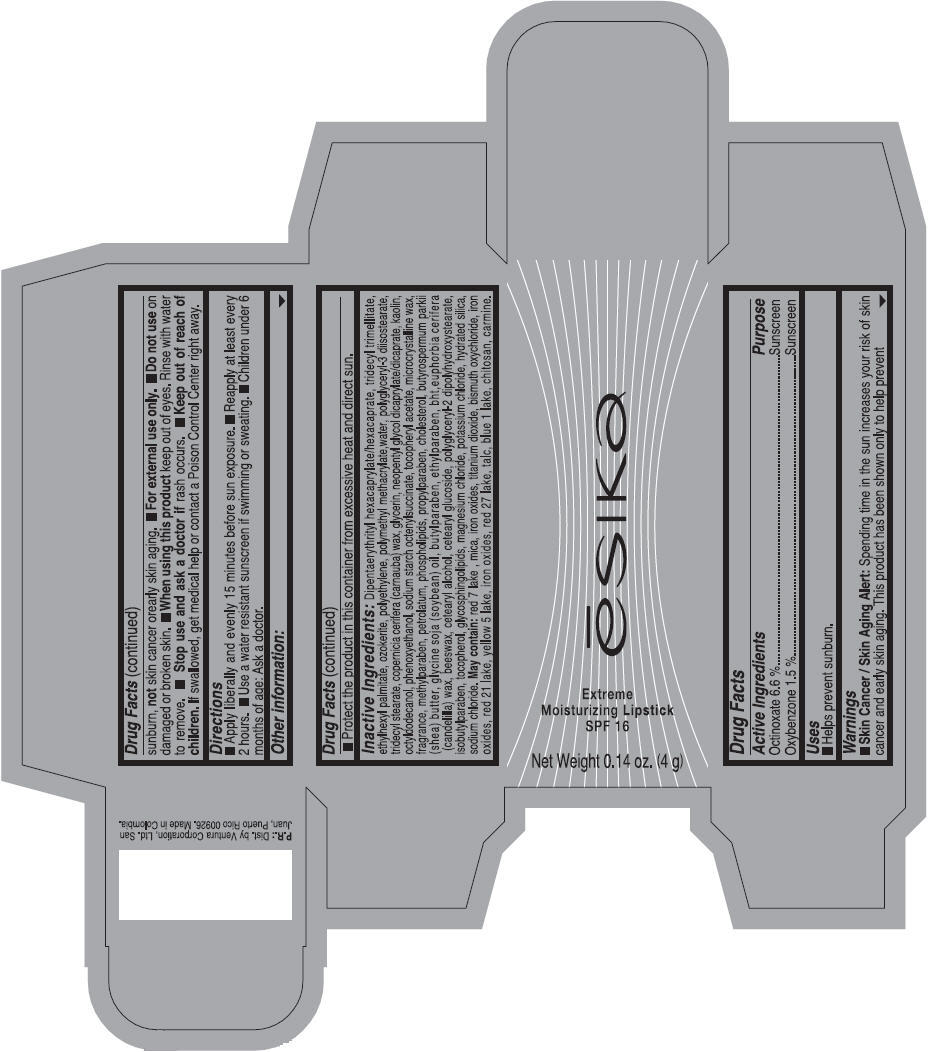
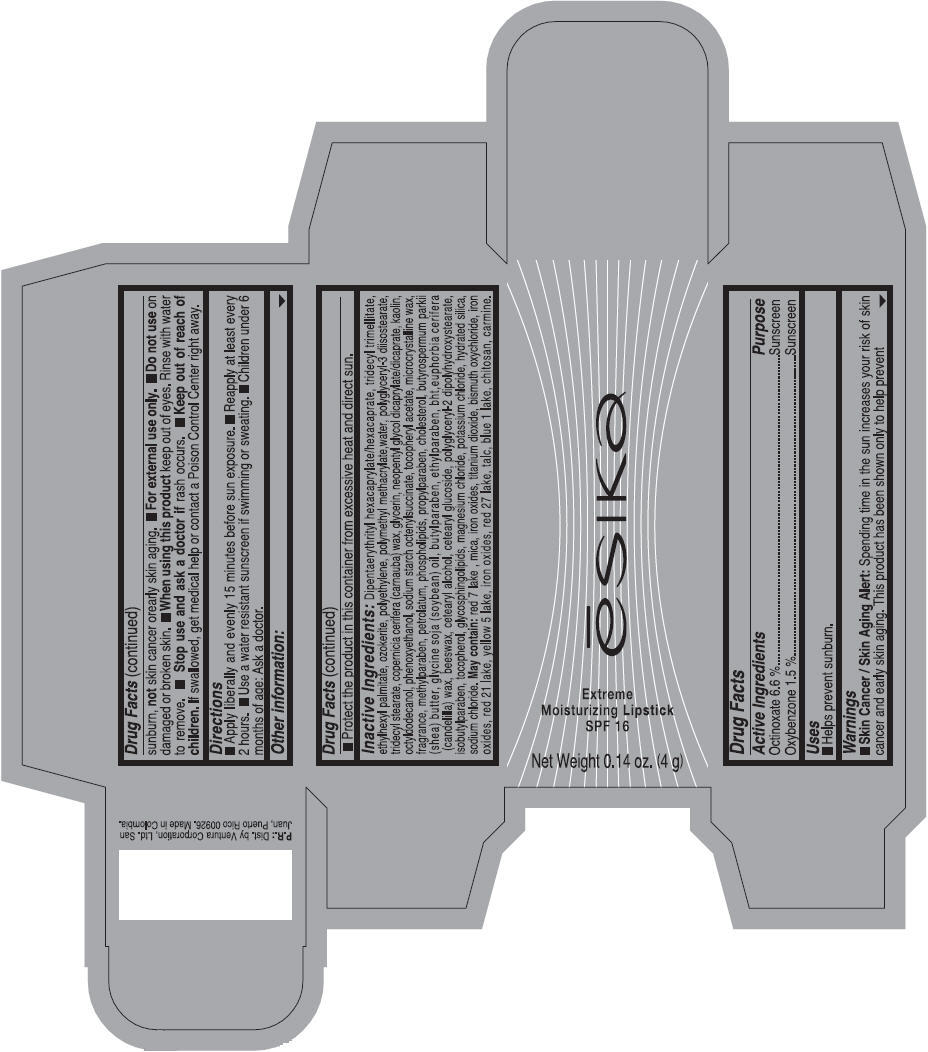
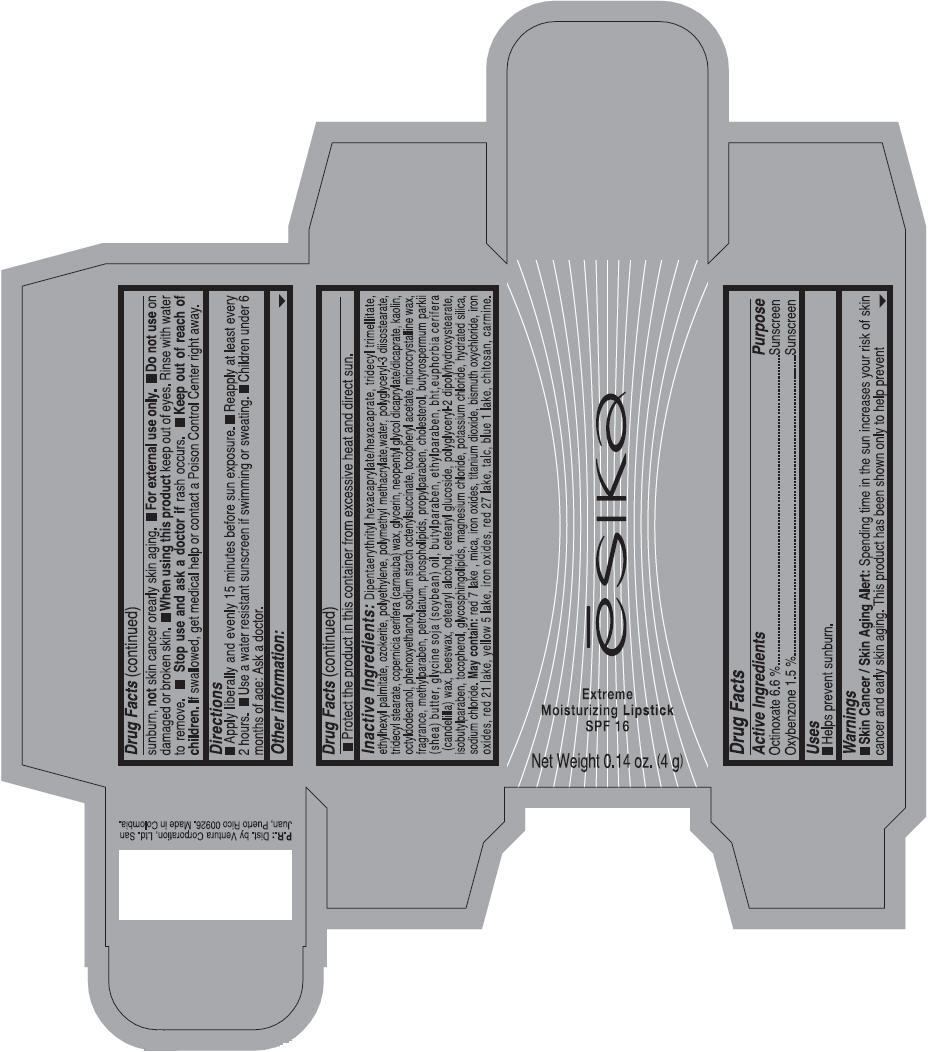
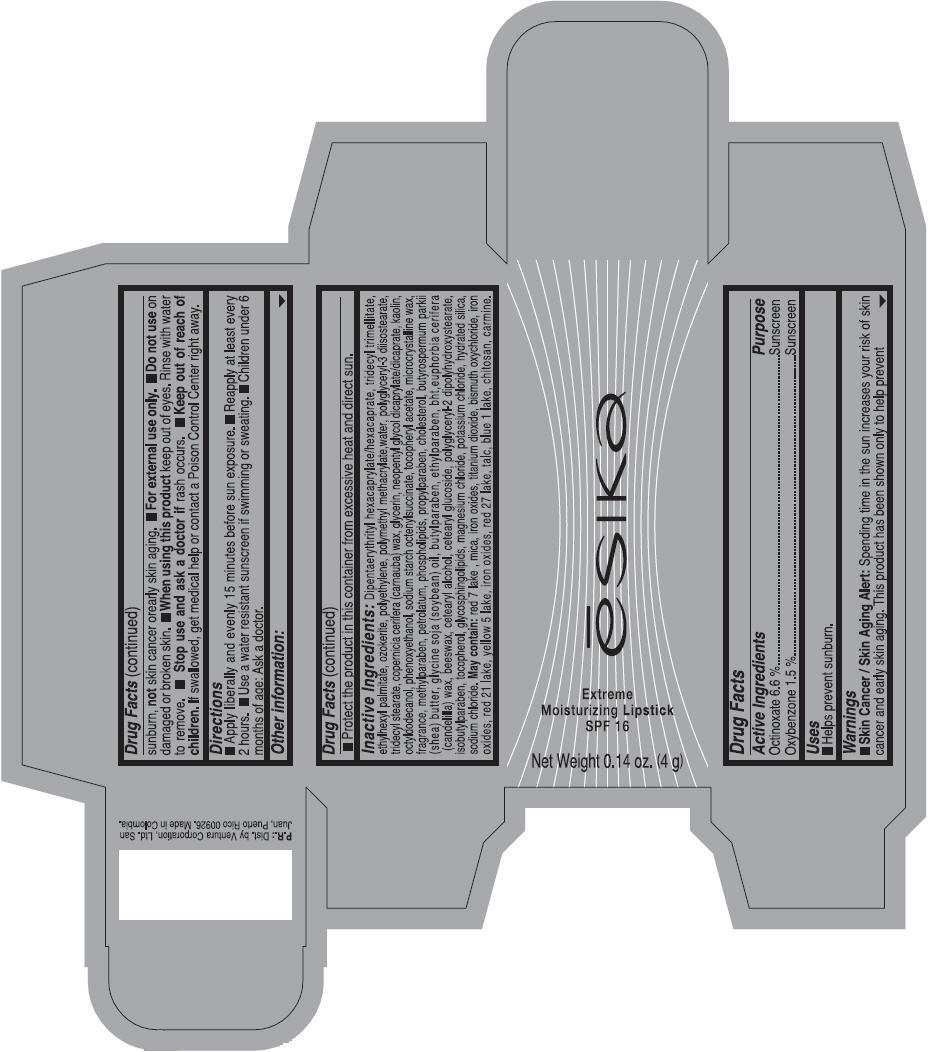
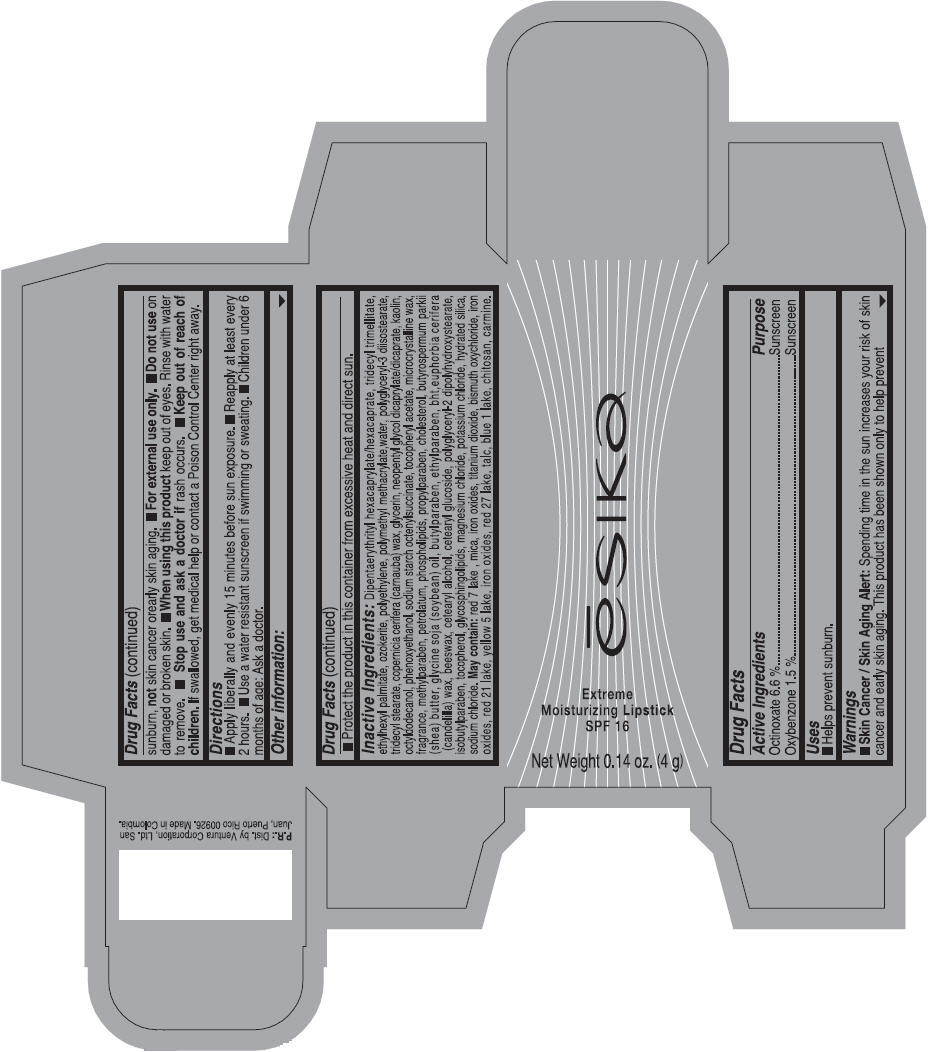
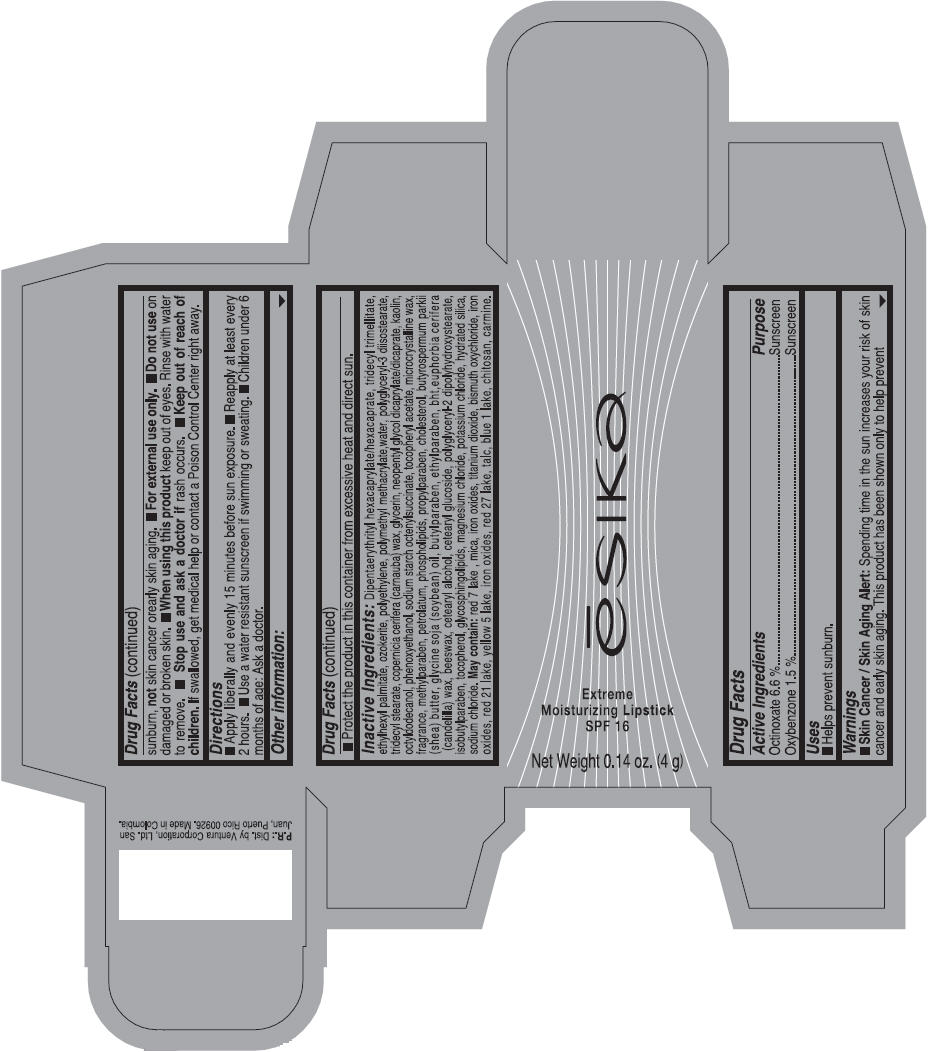
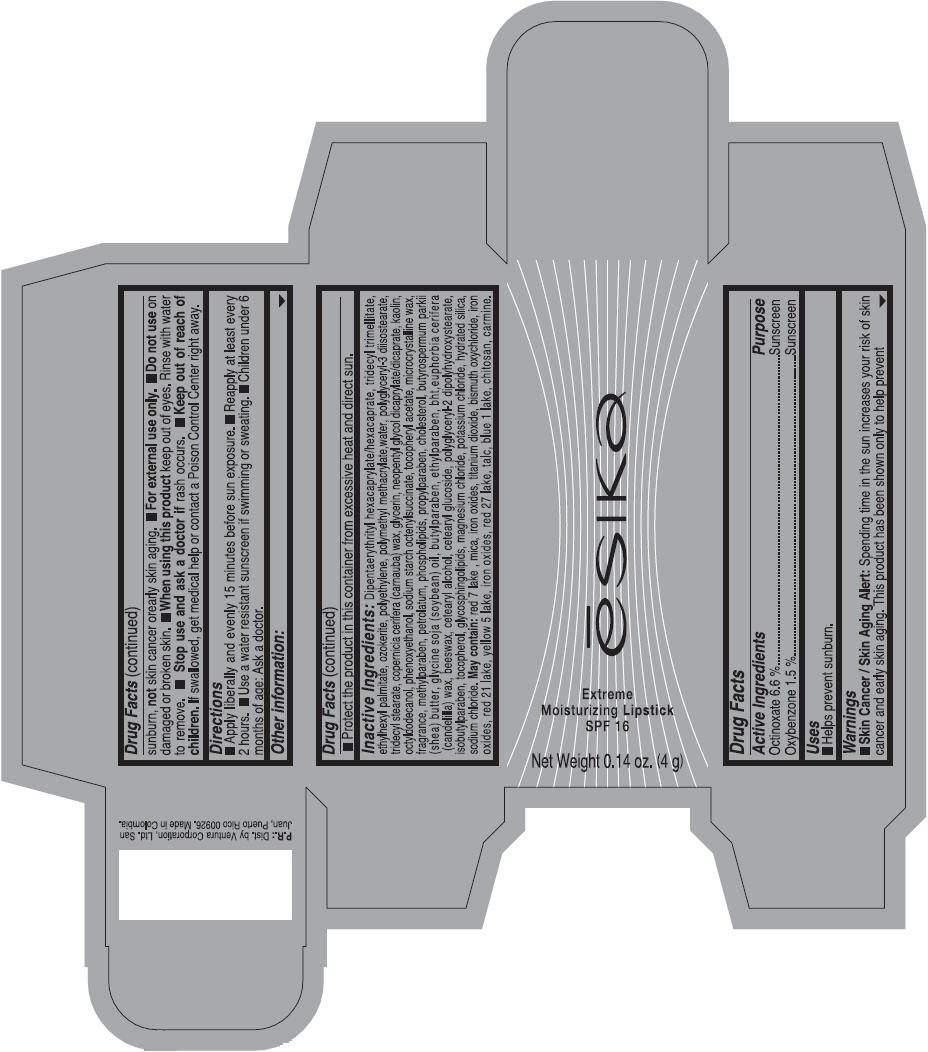
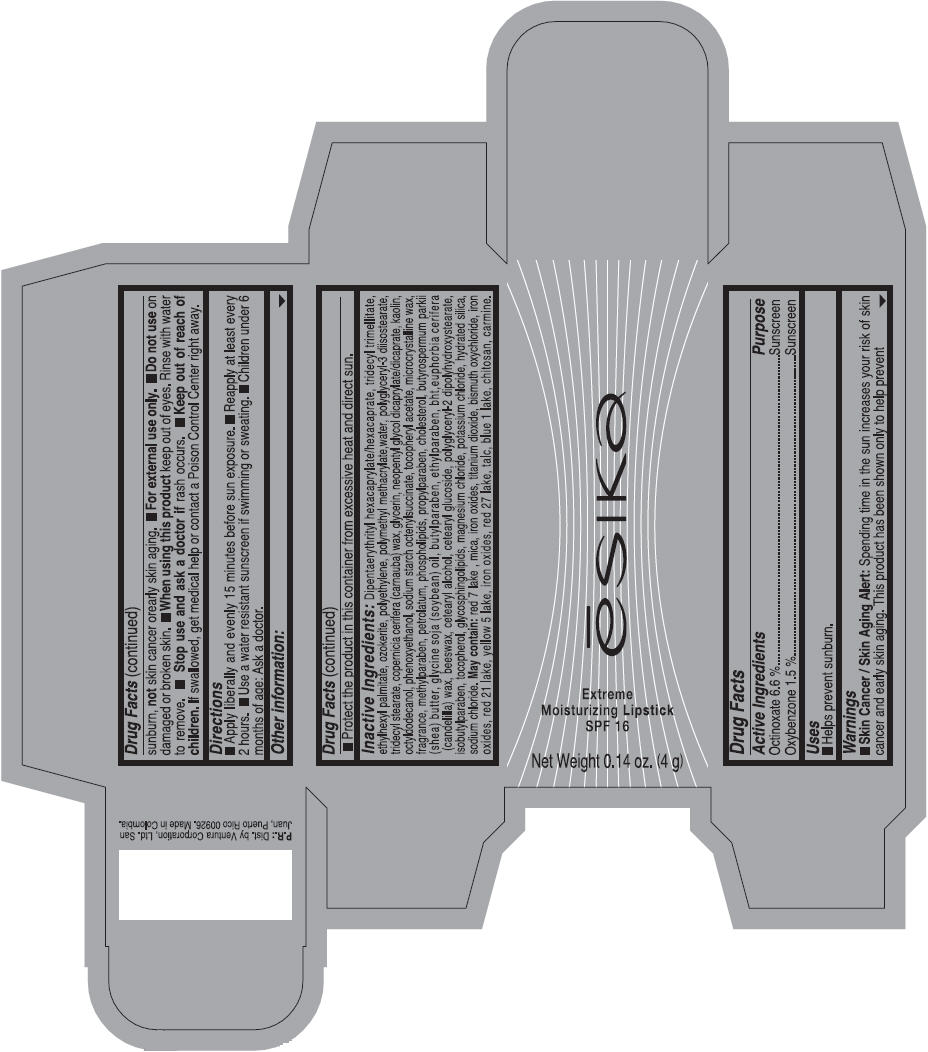
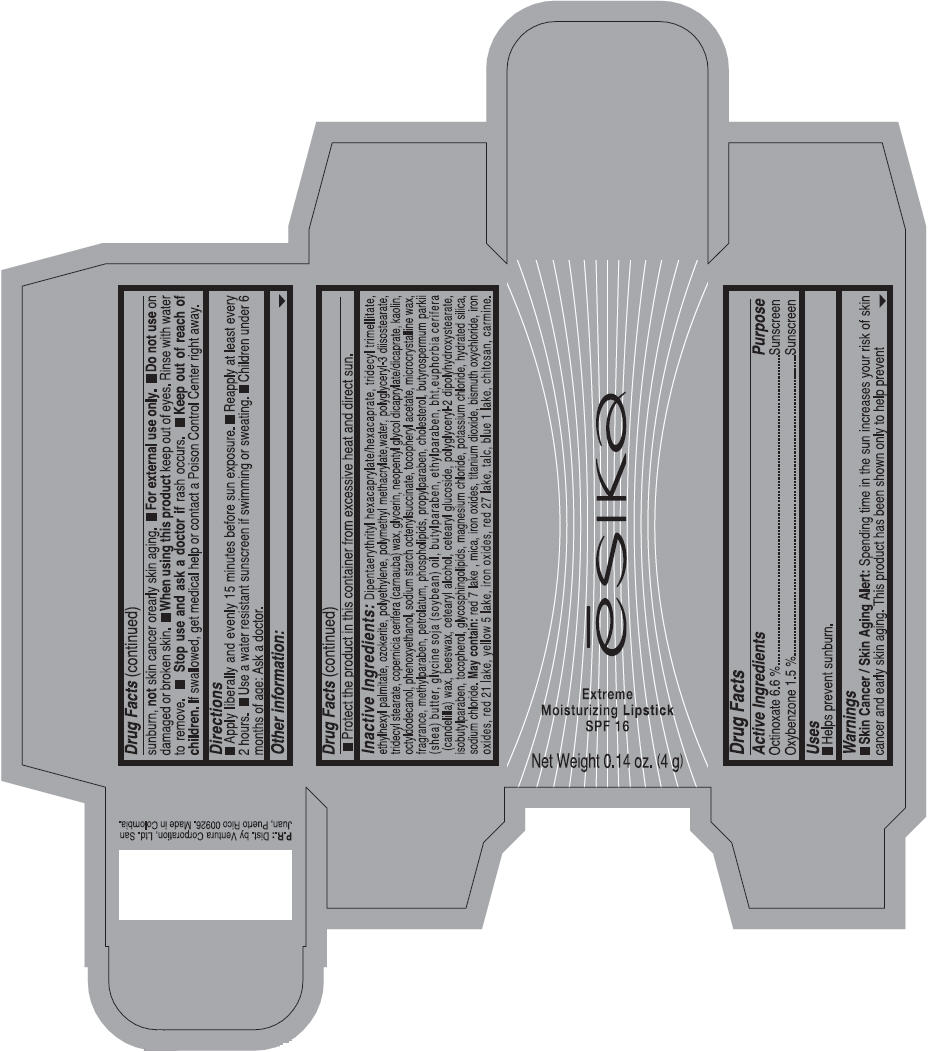
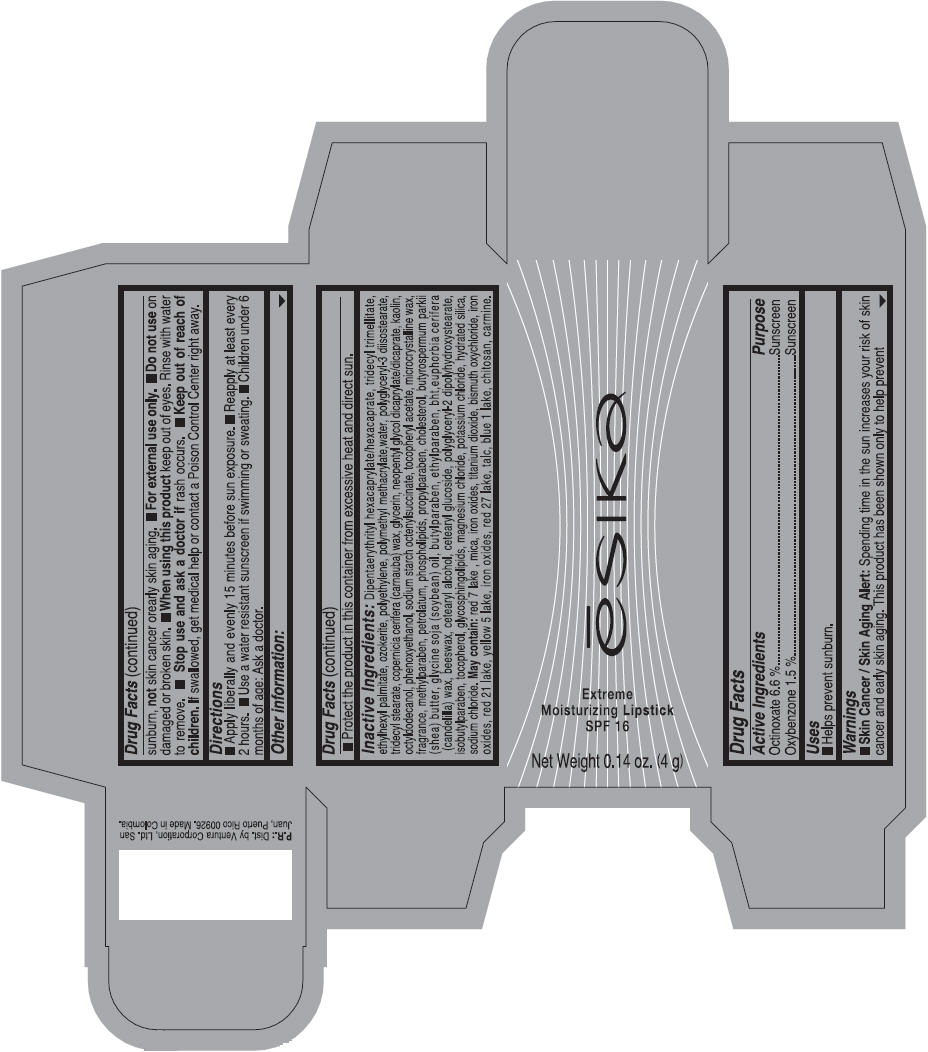
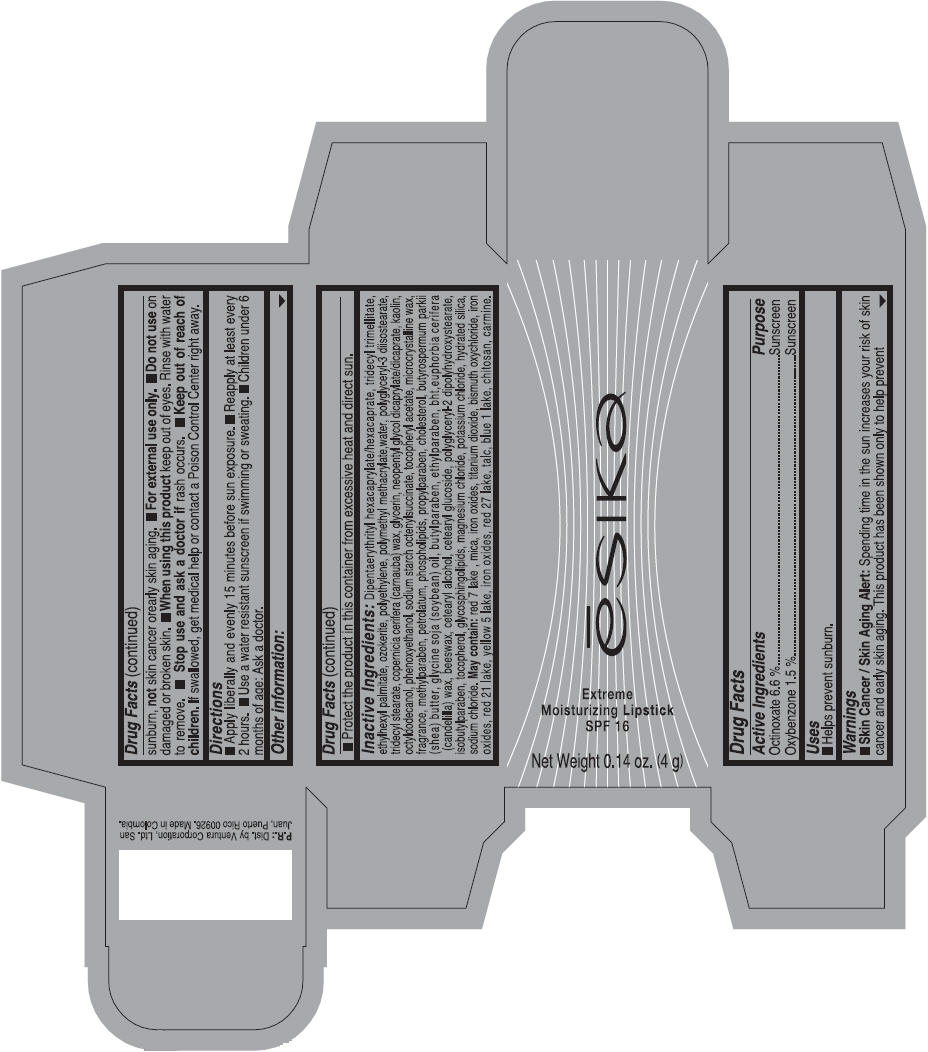
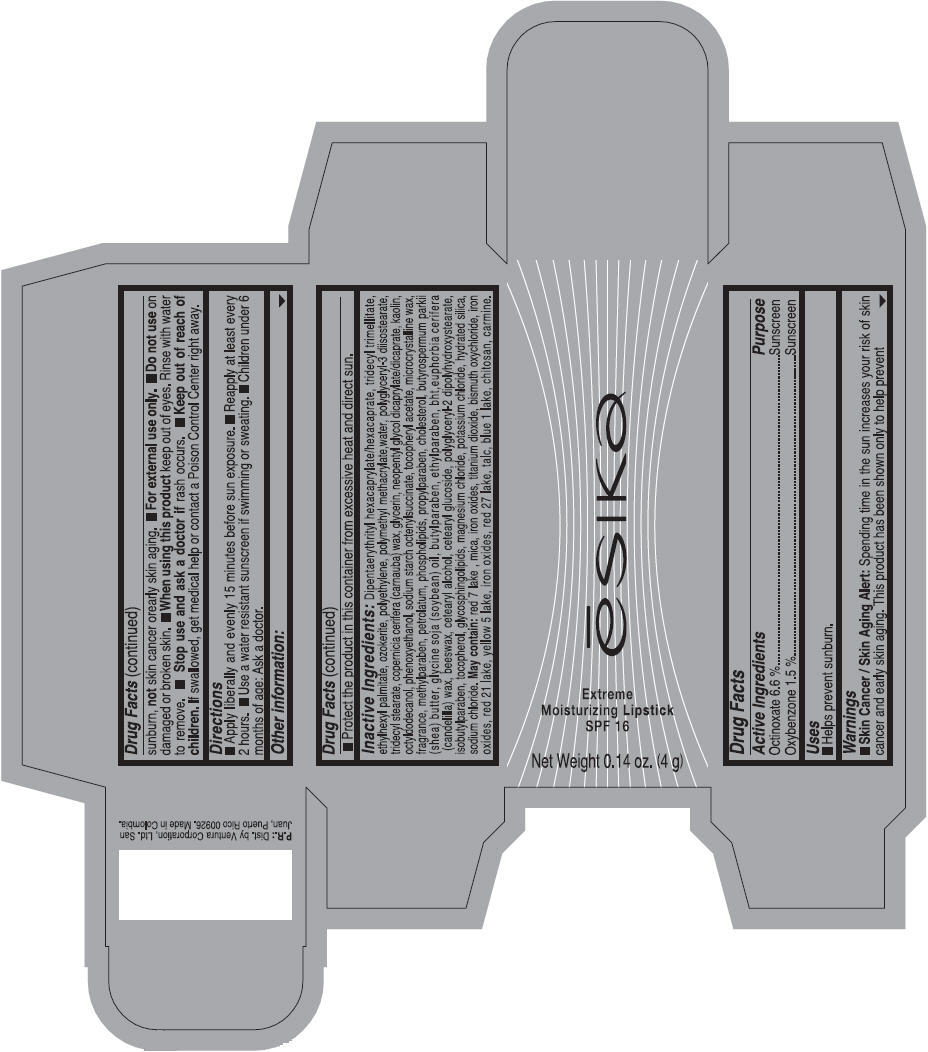
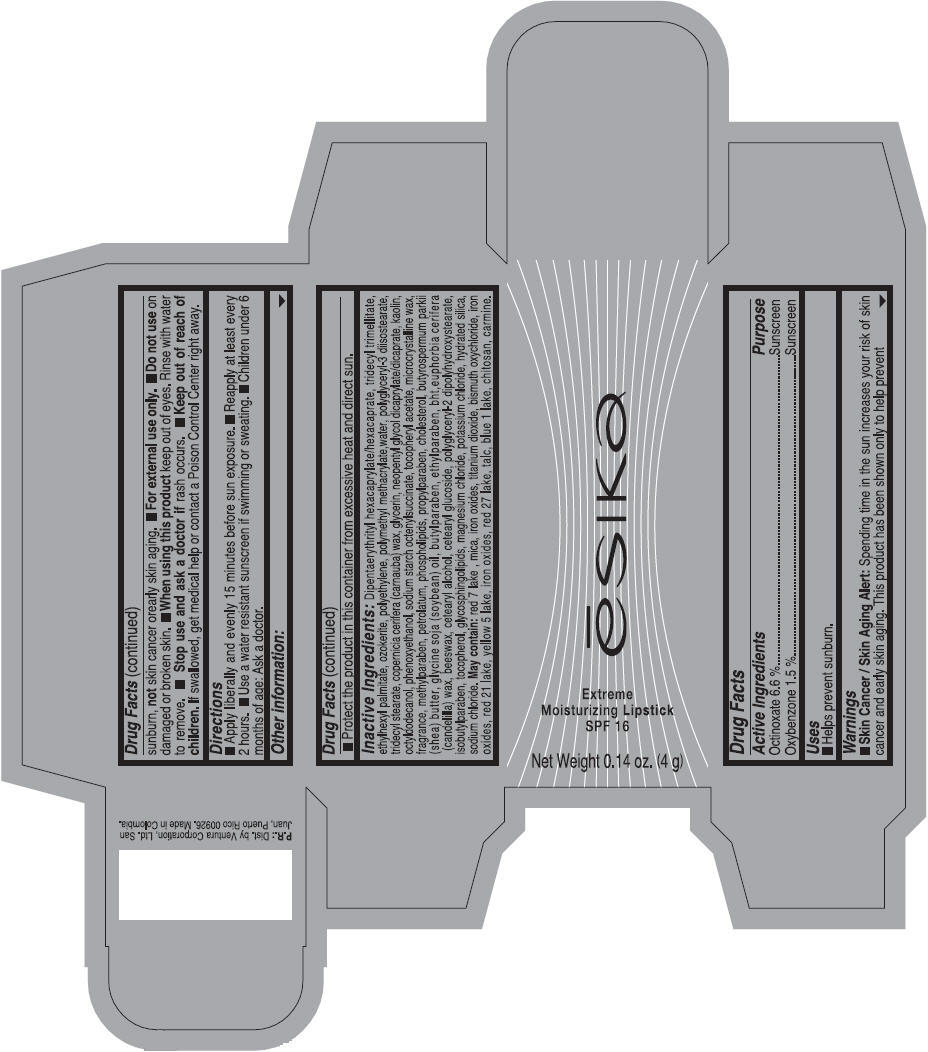
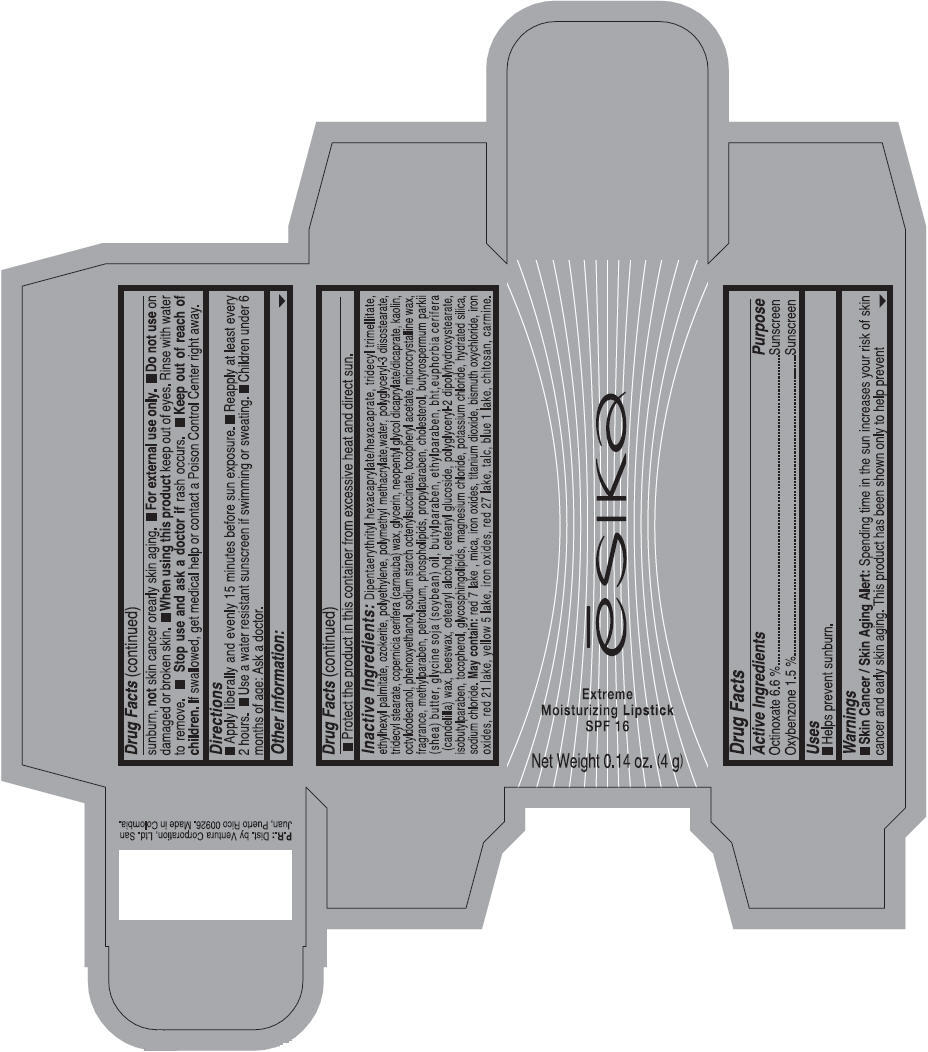
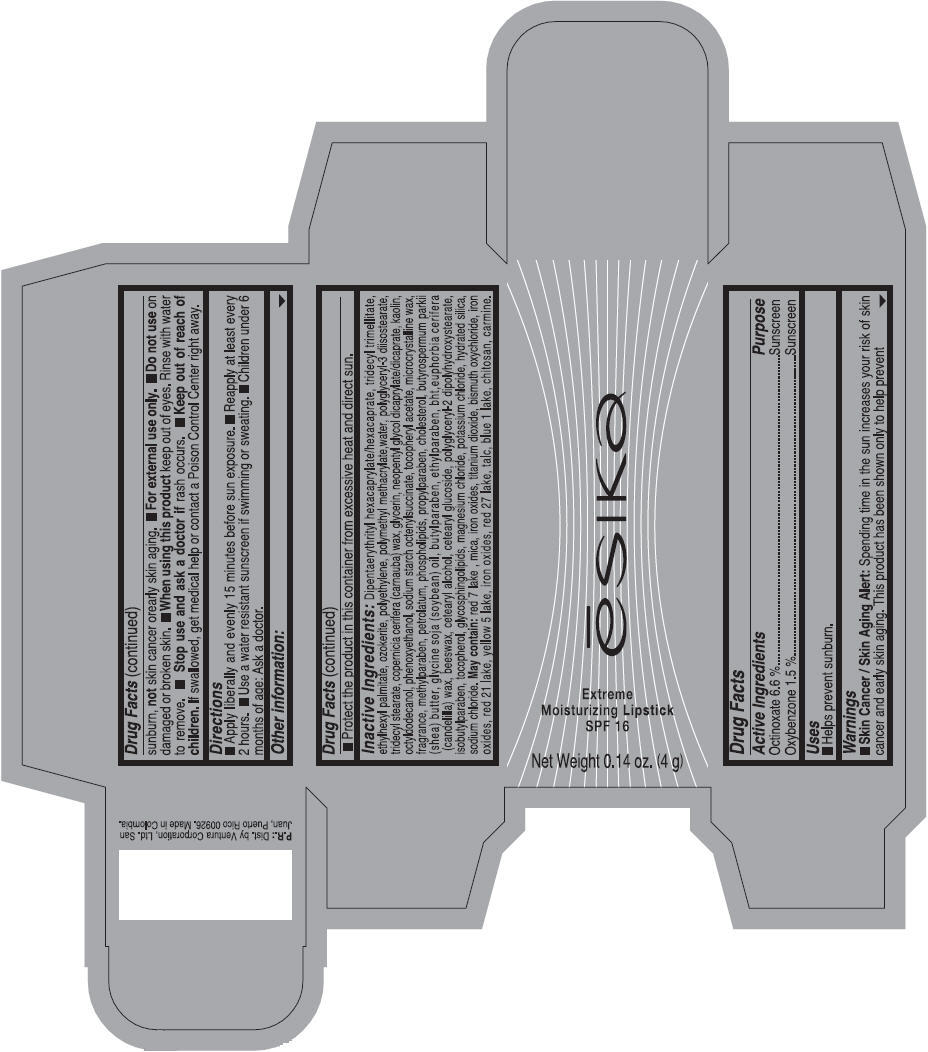
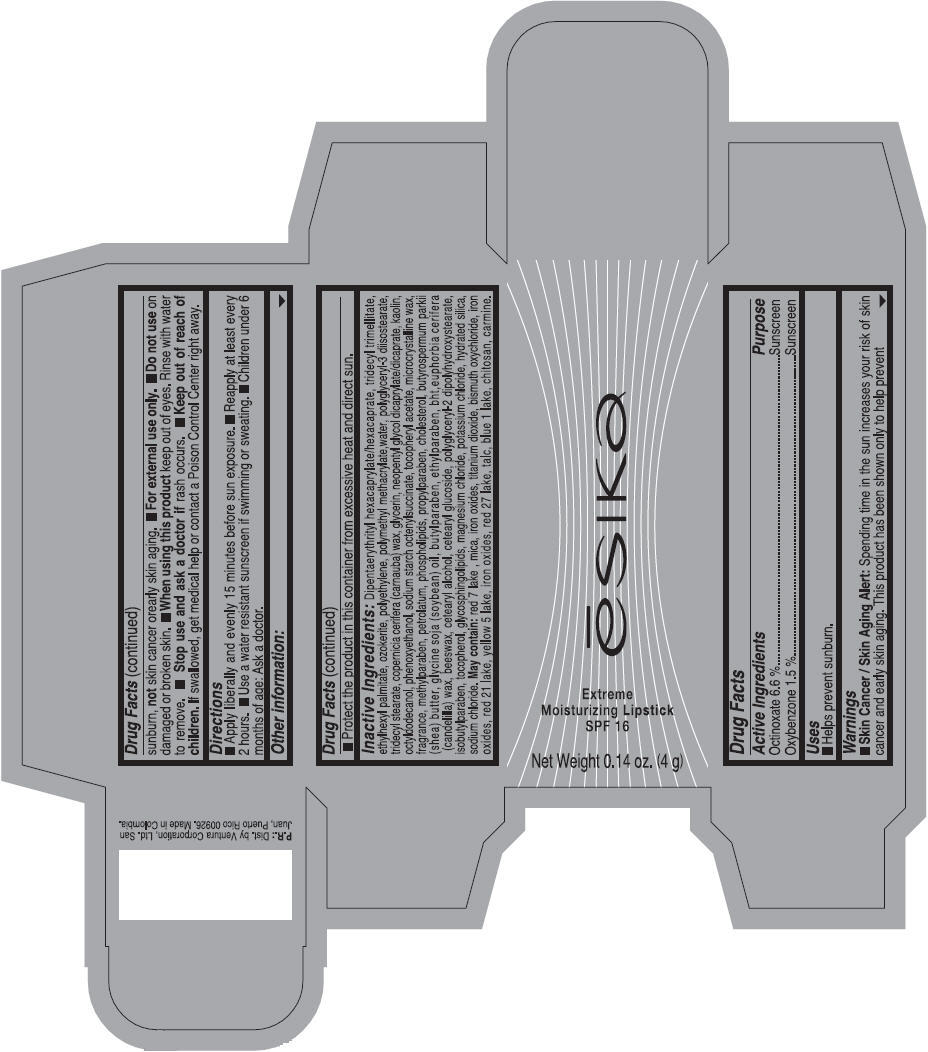
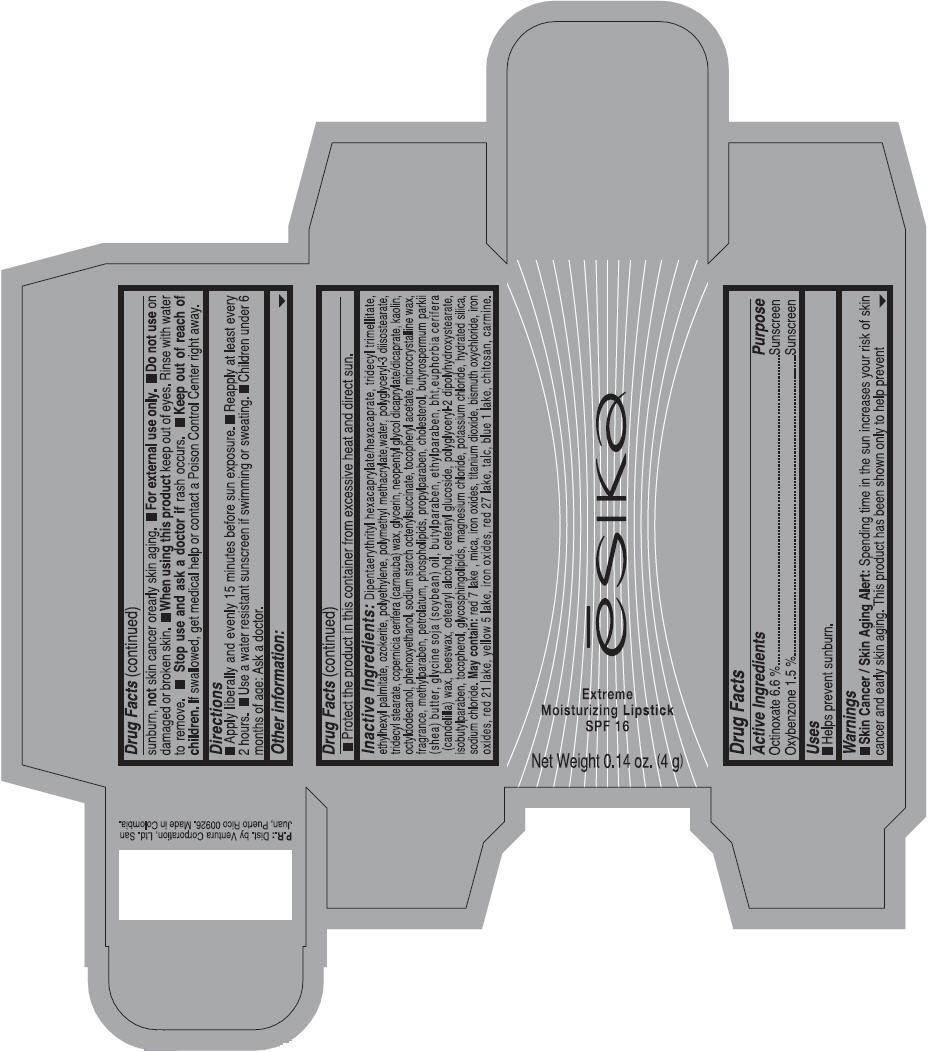
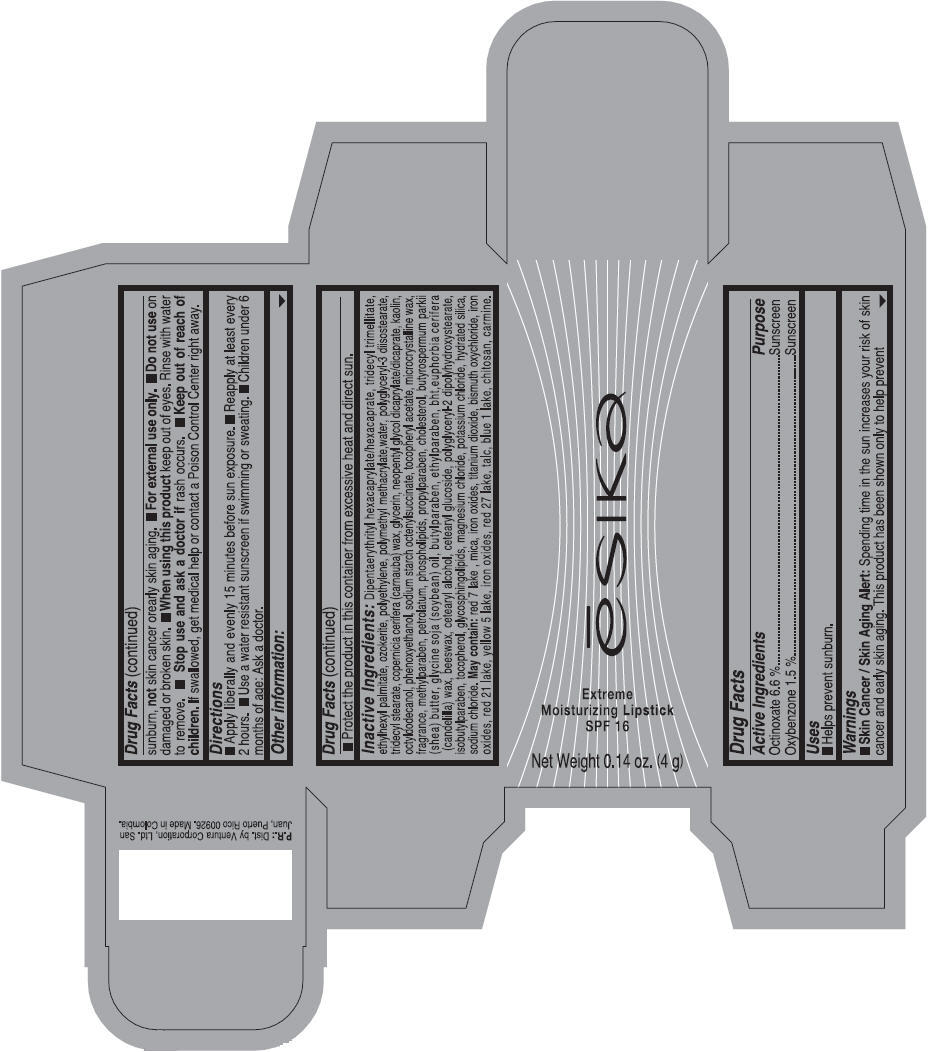
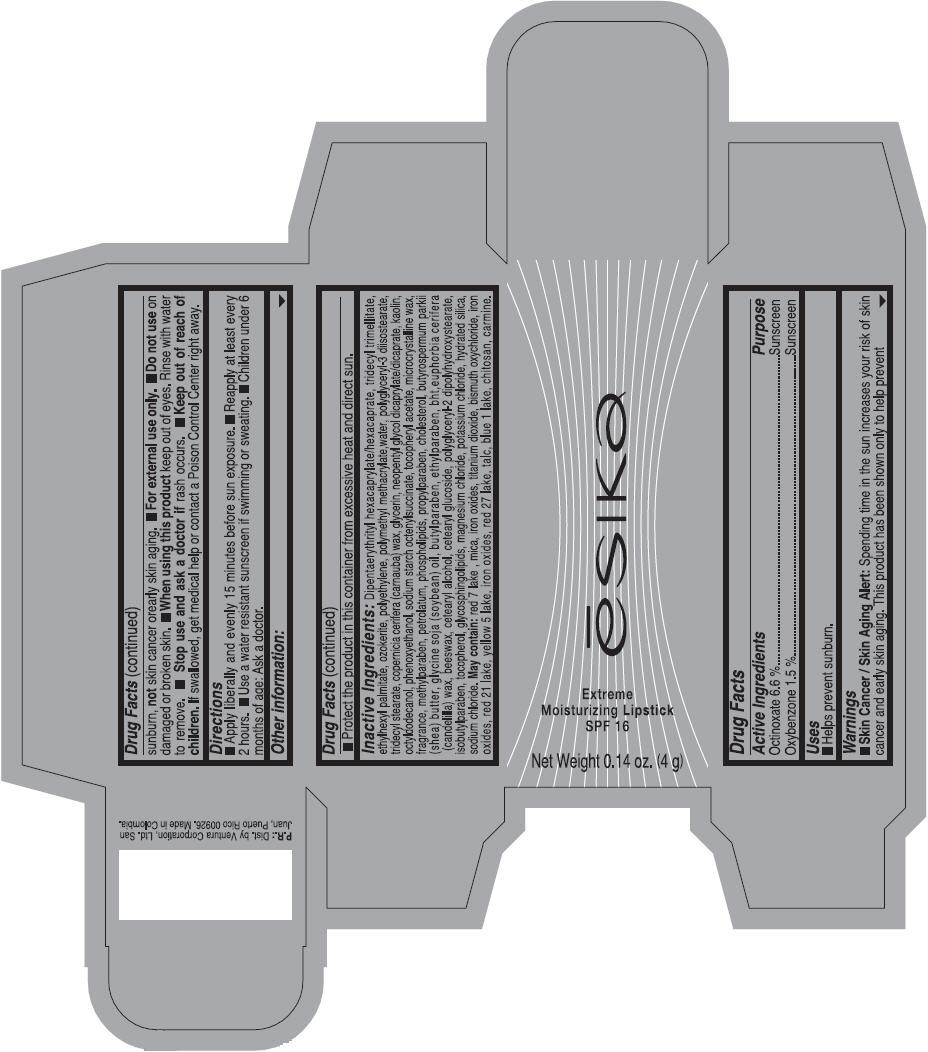
Label: ESIKA EXTREME MOISTURIZING SPF 16 (BORGONA SEXY) - BROWN- octinoxate and oxybenzone lipstick
ESIKA EXTREME MOISTURIZING SPF 16 (CAFE MOCA) - BROWN- octinoxate and oxybenzone lipstick
ESIKA EXTREME MOISTURIZING SPF 16 (CORAL CHIC) - PINK- octinoxate and oxybenzone lipstick
ESIKA EXTREME MOISTURIZING SPF 16 (FUCSIA CAUTIVANTE) - PURPLE (octinoxat ....... SPF 16 (CEREZA HECHIZO) - RED- octinoxate and oxybenzone lipstick
ESIKA EXTREME MOISTURIZING SPF 16 (FRAMBUESA MANIA) - RED- octinoxate and oxybenzone lipstick
ESIKA EXTREME MOISTURIZING SPF 16 (ROSA DULZURA) - PINK- octinoxate and oxybenzone lipstick
ESIKA EXTREME MOISTURIZING SPF 16 (PIMIENTA CALIENTE) - BROWN- octinoxate and oxybenzone lipstick
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-277-01, 13537-277-02, 13537-278-03, 13537-278-04, view more13537-280-07, 13537-280-08, 13537-281-09, 13537-281-10, 13537-282-11, 13537-282-12, 13537-283-13, 13537-283-14, 13537-284-15, 13537-284-16, 13537-285-17, 13537-285-18, 13537-286-19, 13537-286-20, 13537-287-21, 13537-287-22, 13537-288-23, 13537-288-24, 13537-289-25, 13537-289-26, 13537-290-27, 13537-290-28, 13537-291-29, 13537-291-30, 13537-292-31, 13537-292-32, 13537-293-33, 13537-293-34, 13537-294-35, 13537-294-36, 13537-295-37, 13537-295-38, 13537-296-39, 13537-296-40, 13537-297-41, 13537-297-42, 13537-379-05, 13537-379-06 - Packager: Ventura Corporation LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 20, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
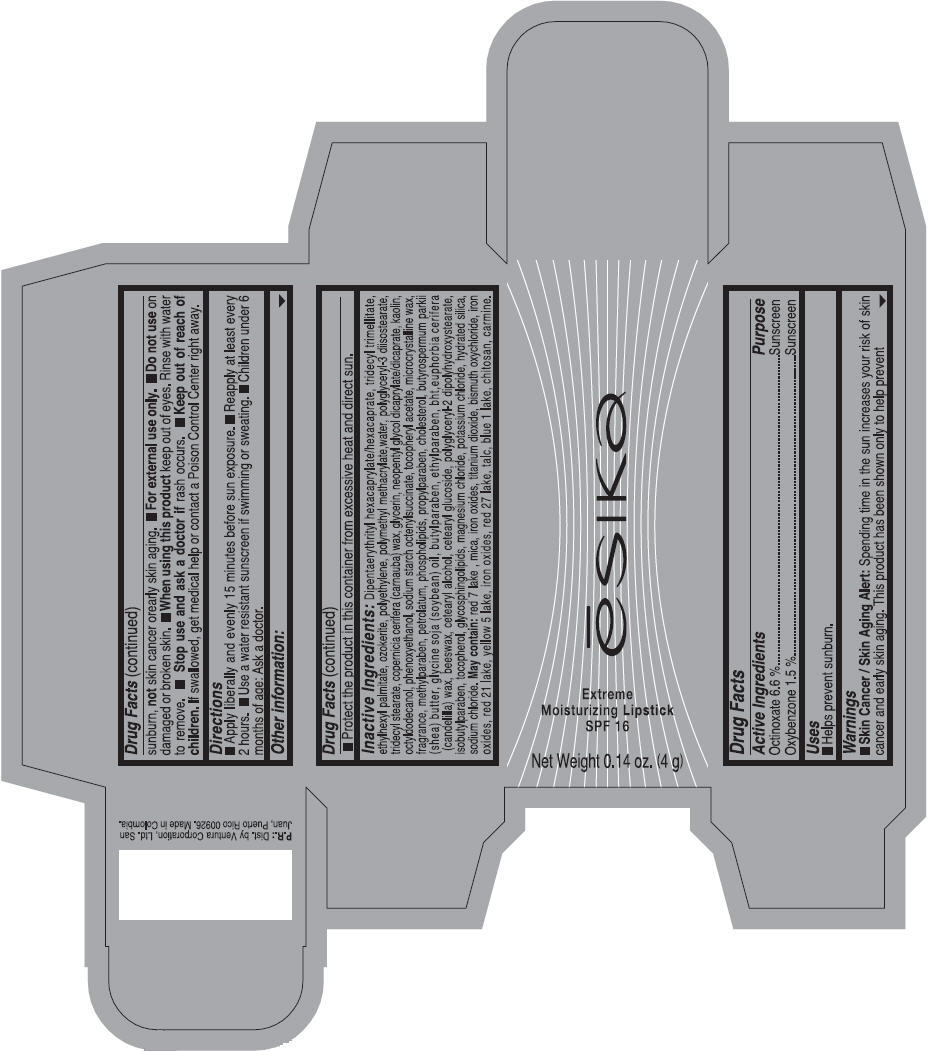
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Dipentaerythrityl hexacaprylate/hexacaprate, tridecyl trimellitate, ethylhexyl palmitate, ozokerite, polyethylene, polymethyl methacrylate,water, polyglyceryl-3 diisostearate, tridecyl stearate, copernicia cerifera (carnauba) wax, glycerin, neopentyl glycol dicaprylate/dicaprate, kaolin, octyldodecanol, phenoxyethanol, sodium starch octenylsuccinate, tocopheryl acetate, microcrystalline wax, fragrance, methylparaben, petrolatum, phospholipids, propylparaben, cholesterol, butyrospermum parkii (shea) butter, glycine soja (soybean) oil, butylparaben, ethylparaben, bht,euphorbia cerifera (candelilla) wax, beeswax, cetearyl alcohol, cetearyl glucoside, polyglyceryl-2 dipolyhydroxystearate, isobutylparaben, tocopherol, glycosphingolipids, magnesium chloride, potassium chloride, hydrated silica, sodium chloride.
May contain: red 7 lake , mica, iron oxides, titanium dioxide, bismuth oxychloride, iron oxides, red 21 lake, yellow 5 lake, iron oxides, red 27 lake , talc, blue 1 lake, chitosan, carmine.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Borgona Sexy - Brown
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Café Moca - Brown
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Coral Chic - Pink
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Fucsia Cautivante - Purple
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Fucsia Glam - Purple
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Granate Seductor - Purple
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Magenta Traviesa - Purple
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Marron Glamour - Brown
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Morado Exuberante - Purple
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Naranja Primavera - Red
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Rojo Deseo - Red
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Rojo Intrigante - Red
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Rojo Lujuria - Red
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Rosa Diva - Red
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Rosa Encanto - Pink
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Rosa Esplendor - Pink
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Rosa Romántica - Pink
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Cereza Hechizo - Red
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Frambuesa Manía - Red
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Rosa Dulzura - Pink
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - Pimienta Caliente - Brown
-
INGREDIENTS AND APPEARANCE
ESIKA EXTREME MOISTURIZING SPF 16 (BORGONA SEXY) - BROWN
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-277 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-277-02 1 in 1 BOX 1 NDC:13537-277-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (CAFE MOCA) - BROWN
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-278 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-278-04 1 in 1 BOX 1 NDC:13537-278-03 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (CORAL CHIC) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-379 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-379-06 1 in 1 BOX 1 NDC:13537-379-05 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (FUCSIA CAUTIVANTE) - PURPLE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-280 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-280-08 1 in 1 BOX 1 NDC:13537-280-07 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (FUCSIA GLAM) - PURPLE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-281 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-281-10 1 in 1 BOX 1 NDC:13537-281-09 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (GRANATE SEDUCTOR) - PURPLE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-282 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-282-12 1 in 1 BOX 1 NDC:13537-282-11 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (MAGENTA TRAVIESA) - PURPLE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-283 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-283-14 1 in 1 BOX 1 NDC:13537-283-13 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (MARRON GLAMOUR) - BROWN
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-284 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-284-16 1 in 1 BOX 1 NDC:13537-284-15 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (MORADO EXUBERANTE) - PURPLE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-285 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-285-18 1 in 1 BOX 1 NDC:13537-285-17 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (NARANJA PRIMAVERA) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-286 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-286-20 1 in 1 BOX 1 NDC:13537-286-19 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (ROJO DESEO) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-287 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-287-22 1 in 1 BOX 1 NDC:13537-287-21 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (ROJO INTRIGANTE) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-288 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-288-24 1 in 1 BOX 1 NDC:13537-288-23 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (ROJO LUJURIA) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-289 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-289-26 1 in 1 BOX 1 NDC:13537-289-25 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (ROSA DIVA) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-290 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-290-28 1 in 1 BOX 1 NDC:13537-290-27 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (ROSA ENCANTO) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-291 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-291-30 1 in 1 BOX 1 NDC:13537-291-29 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (ROSA ESPLENDOR) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-292 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-292-32 1 in 1 BOX 1 NDC:13537-292-31 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (ROSA ROMANTICA) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-293 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-293-34 1 in 1 BOX 1 NDC:13537-293-33 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (CEREZA HECHIZO) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-294 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-294-36 1 in 1 BOX 1 NDC:13537-294-35 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (FRAMBUESA MANIA) - RED
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-295 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-295-38 1 in 1 BOX 1 NDC:13537-295-37 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (ROSA DULZURA) - PINK
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-296 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) cetearyl glucoside (UNII: 09FUA47KNA) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-296-40 1 in 1 BOX 1 NDC:13537-296-39 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 ESIKA EXTREME MOISTURIZING SPF 16 (PIMIENTA CALIENTE) - BROWN
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-297 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.066 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.015 g in 1 g Inactive Ingredients Ingredient Name Strength Dipentaerythrityl hexacaprylate/hexacaprate (UNII: 554N82UWVW) tridecyl trimellitate (UNII: FY36J270ES) ethylhexyl palmitate (UNII: 2865993309) high density polyethylene (UNII: UG00KM4WR7) water (UNII: 059QF0KO0R) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) tridecyl stearate (UNII: A8OE252M6L) carnauba wax (UNII: R12CBM0EIZ) glycerin (UNII: PDC6A3C0OX) neopentyl glycol dicaprylate/dicaprate (UNII: VLW429K27K) kaolin (UNII: 24H4NWX5CO) octyldodecanol (UNII: 461N1O614Y) phenoxyethanol (UNII: HIE492ZZ3T) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) microcrystalline wax (UNII: XOF597Q3KY) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) propylparaben (UNII: Z8IX2SC1OH) cholesterol (UNII: 97C5T2UQ7J) shea butter (UNII: K49155WL9Y) soybean oil (UNII: 241ATL177A) butylparaben (UNII: 3QPI1U3FV8) ethylparaben (UNII: 14255EXE39) butylated hydroxytoluene (UNII: 1P9D0Z171K) candelilla wax (UNII: WL0328HX19) yellow wax (UNII: 2ZA36H0S2V) cetostearyl alcohol (UNII: 2DMT128M1S) polyglyceryl-2 dipolyhydroxystearate (UNII: 9229XJ4V12) cetearyl glucoside (UNII: 09FUA47KNA) isobutylparaben (UNII: 0QQJ25X58G) tocopherol (UNII: R0ZB2556P8) magnesium chloride (UNII: 02F3473H9O) potassium chloride (UNII: 660YQ98I10) hydrated silica (UNII: Y6O7T4G8P9) sodium chloride (UNII: 451W47IQ8X) d&c red no. 7 (UNII: ECW0LZ41X8) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) bismuth oxychloride (UNII: 4ZR792I587) d&c red no. 21 (UNII: 08744Z6JNY) fd&c yellow no. 5 (UNII: I753WB2F1M) d&c red no. 27 (UNII: 2LRS185U6K) fd&c blue no. 1 (UNII: H3R47K3TBD) talc (UNII: 7SEV7J4R1U) aluminum oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-297-42 1 in 1 BOX 1 NDC:13537-297-41 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/16/2013 Labeler - Ventura Corporation LTD (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-277, 13537-278, 13537-379, 13537-280, 13537-281, 13537-282, 13537-283, 13537-284, 13537-285, 13537-286, 13537-287, 13537-288, 13537-289, 13537-290, 13537-291, 13537-292, 13537-293, 13537-294, 13537-295, 13537-296, 13537-297)