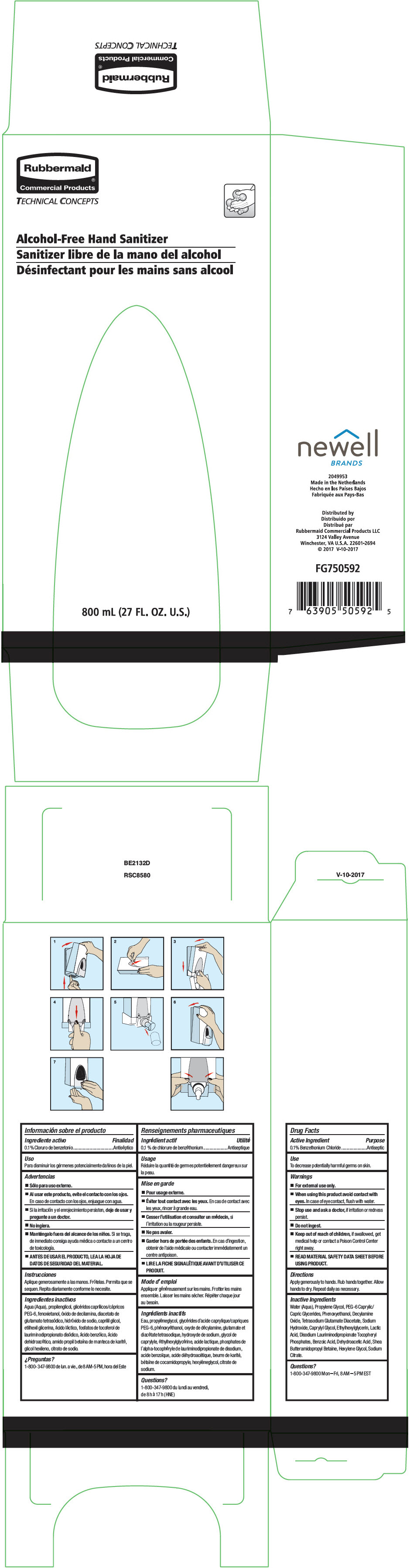
Label: TC ALCOHOL-FREE HAND SANITIZER- benzethonium chloride liquid
- NDC Code(s): 65321-034-01, 65321-034-02
- Packager: Rubbermaid Commercial Products LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 29, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
-
Inactive Ingredients
Water (Aqua), Propylene Glycol, PEG-6 Caprylic/Capric Glycerides, Phenoxyethanol, Decylamine Oxide, Tetrasodium Glutamate Diacetate, Sodium Hydroxide, Caprylyl Glycol, Ethylhexylglycerin, Lactic Acid, Disodium Lauriminodipropionate Tocopheryl Phosphates, Benzoic Acid, Dehydroacetic Acid, Shea Butteramidopropyl Betaine, Hexylene Glycol, Sodium Citrate.
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 800 mL Pouch Box
-
INGREDIENTS AND APPEARANCE
TC ALCOHOL-FREE HAND SANITIZER
benzethonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65321-034 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 0.1 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CAPRYLOCAPROYL POLYOXYLGLYCERIDES 6 (UNII: GO50W2HWO8) PHENOXYETHANOL (UNII: HIE492ZZ3T) DECYLAMINE OXIDE (UNII: G387VUT5EZ) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) SODIUM HYDROXIDE (UNII: 55X04QC32I) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) DISODIUM LAURIMINODIPROPIONATE TOCOPHERYL PHOSPHATES (UNII: 0K5Y9U1P6M) BENZOIC ACID (UNII: 8SKN0B0MIM) DEHYDROACETIC ACID (UNII: 2KAG279R6R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65321-034-01 4 in 1 BOX 10/28/2019 10/12/2023 1 800 mL in 1 POUCH; Type 0: Not a Combination Product 2 NDC:65321-034-02 4 in 1 BOX 10/28/2019 10/12/2023 2 1100 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M004 10/28/2019 Labeler - Rubbermaid Commercial Products LLC (049924368) Establishment Name Address ID/FEI Business Operations NWL Netherlands Production BV 489421698 ANALYSIS(65321-034) , MANUFACTURE(65321-034)