Label: NATURAL FX- octinoxate, octocrylene, oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 68327-002-01 - Packager: Cover FX Skin Care, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 20, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients Section
- Purpose Section
- Keep Out Of Reach Of Children Section
- Uses
-
Warning Section
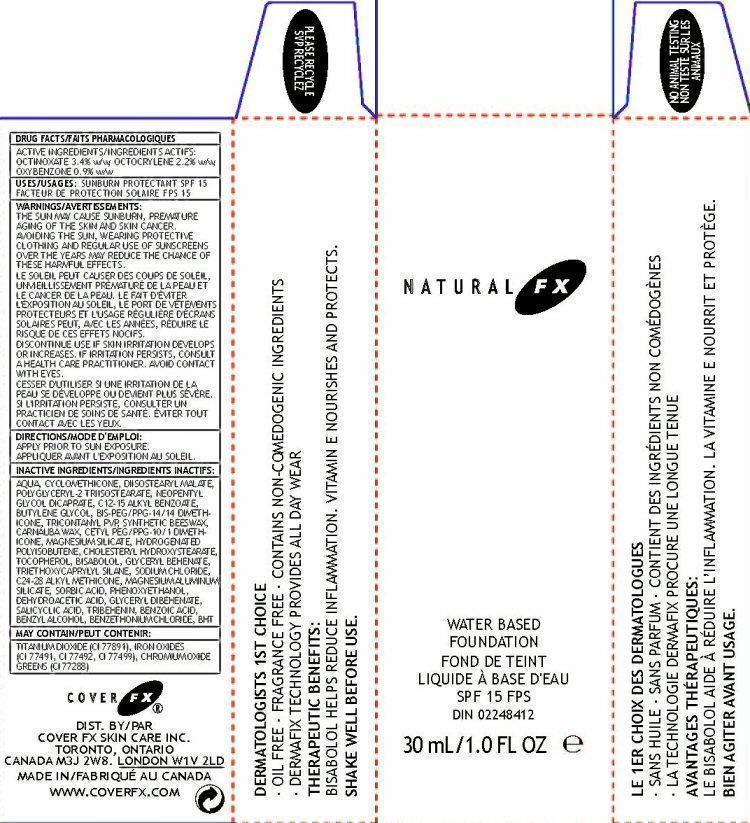
WARNINGS:
THE SUN MAY CAUSE SUNBURN, PREMATURE AGING OF THE SKIN AND SKIN CANCER. AVOIDING THE SUN, WEARING PROTECTIVE CLOTHING AND REGULAR USE OF SUNSCREEN OVER THE YEARS MAY REDUCE THE CHANCE OF THESE HARMFUL EFFECTS.
DISCONTINUE USE IF SKIN IRRITATION DEVELOPS OR INCREASES. IF SKIN IRRITATION PERSISTS, CONTACT A HEALTH CARE PRACTITIONER. AVOID CONTACT WITH EYES.
- Directions
-
Inactive Ingredients
INACTIVE INGREDIENTS:
WATER, CYCLOMETHICONE, DIIOSTEARYL MALATE,POLYGLYCERYL-2 TRIISOSTEARATE, NEOPENTYL GLYCOL DICAPRATE, C12-15 ALKYL BENZOATE, BUTYLENE GLYCOL, BIS-PEG/PPG-14/14 DIMETHICONE,TRICONTANYL PVP, SYNTHETIC BEESWAX,CARNAUBA WAX, CETYL PEG/PPG-10/1 DIMETHICONE, MAGNESIUM SILICATE, HYDROGENATED POLYISOBUTENE, CHOLESTERYL HYDROXYSTEARATE,
TOCOPHEROL, BISABOLOL, GLYCERYL BEHENATE, TRIETHOXYCAPRYLYL SILANE, SODIUM CHLORIDE,C24-28 ALKYL METHICONE, MAGNESIUM ALUMINUM SILICATE, SORBIC ACID, PHENOXYETHANOL, DEHYDROACETIC ACID, GLYCERYL DIBEHENATE,
SALICYCLIC ACID, TRIBEHENIN, BENZOIC ACID, BENZYL ALCOHOL, BENZETHONIUM CHLORIDE, BHTMAY CONTAIN:
TITANIUM DIOXIDE (CI 77891), IRON OXIDES (CI 77491, CI 77492, CI 77499), CHROMIUM OXIDE GREENS (CI 77288)
-
Product Label
NATURAL FX
WATER BASED
FOUNDATION
SPF 15 FPS
DIN 0224841230Ml/1.0 fl oz
WATER FRESH FOUNDATION LEAVES SKIN REHYDRATED AND RADIENT LOOKING. REVOLUUTIONARY DERMAFIX TECHNOLOGY PROVIDES FLEXIBLE COVERAGE AND ALL DAY WEAR. ENRICHED WITH VITAMIN e TO NOURISH AND PROTECT SKIN FROM DAMAGING FREE RADICALS.
WARNING: CONTAINS BENZOPHENONE-3 PLEASE RECYCLE
COVER FX
DIST. BY/PAR
COVER FX SKIN CARE INC.
TORONTO, ONTARIO
CANADA M3J 2W8. LONDON W1V 2LD
MADE IN/FABRIQUÉ AU CANADA
WWW.COVERFX.COMDERMATOLOGISTS 1ST CHOICE
∙ OIL FREE ∙ FRAGRANCE FREE ∙ CONTAINS NON-COMEDOGENIC INGREDIENTS
∙ DERMAFIX TECHNOLOGY PROVIDES ALL DAY WEAR
THERAPEUTIC BENEFITS:
BISABOLOL HELPS REDUCE INFLAMMATION. VITAMIN E NOURISHES AND PROTECTS.
SHAKE WELL BEFORE USE.
YOU MAY REPORT A SERIOUS ADVERSE AFFECT FROM USE OF THIS PRODUCT TO (USA) COVER FX 33 ELM STREET, SUITE 377, CHAMPLANE, NY 12919
(CANADA) COVER FX, 1681 FLINT RD, TORONTO, ON M3J 2W8

-
INGREDIENTS AND APPEARANCE
NATURAL FX
octinoxate, octocrylene, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68327-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.4 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.2 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 0.9 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE (UNII: NMQ347994Z) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) NEOPENTYL GLYCOL DICAPRATE (UNII: 77T908SE82) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TRIACONTANYL PVP (WP-660) (UNII: N0SS3Q238D) CARNAUBA WAX (UNII: R12CBM0EIZ) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) MAGNESIUM SILICATE (UNII: 9B9691B2N9) HYDROGENATED POLYBUTENE (370 MW) (UNII: V5H8103878) CHOLESTERYL HYDROXYSTEARATE (UNII: O6K1LG6N4D) TOCOPHEROL (UNII: R0ZB2556P8) LEVOMENOL (UNII: 24WE03BX2T) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) SORBIC ACID (UNII: X045WJ989B) PHENOXYETHANOL (UNII: HIE492ZZ3T) DEHYDROACETIC ACID (UNII: 2KAG279R6R) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) SALICYLIC ACID (UNII: O414PZ4LPZ) TRIBEHENIN (UNII: 8OC9U7TQZ0) BENZOIC ACID (UNII: 8SKN0B0MIM) BENZYL ALCOHOL (UNII: LKG8494WBH) BENZETHONIUM CHLORIDE (UNII: PH41D05744) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) CHROMIC OXIDE (UNII: X5Z09SU859) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68327-002-01 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 08/01/2012 Labeler - Cover FX Skin Care, Inc. (202908021) Registrant - Cover FX Skin Care, Inc. (202908021)


