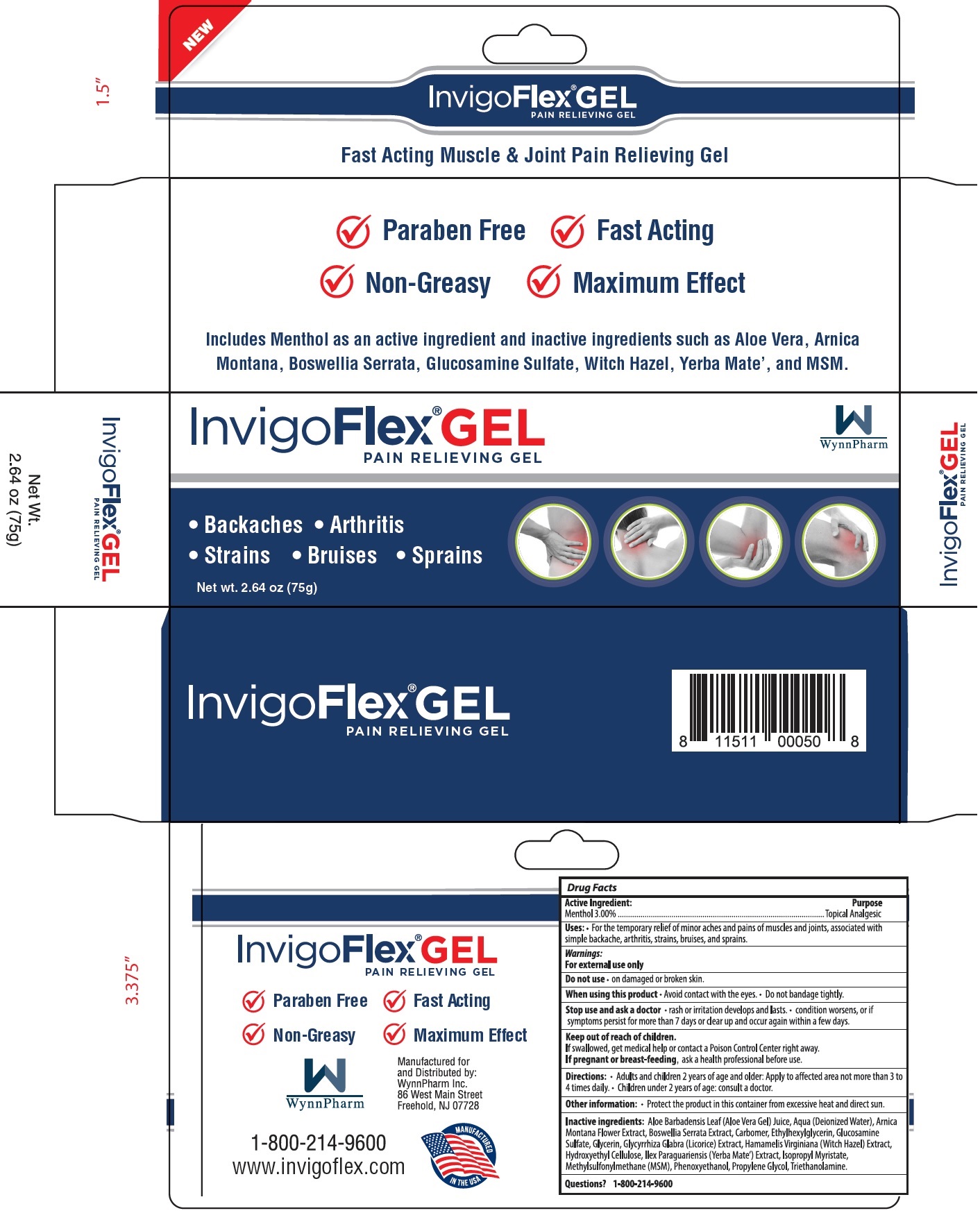
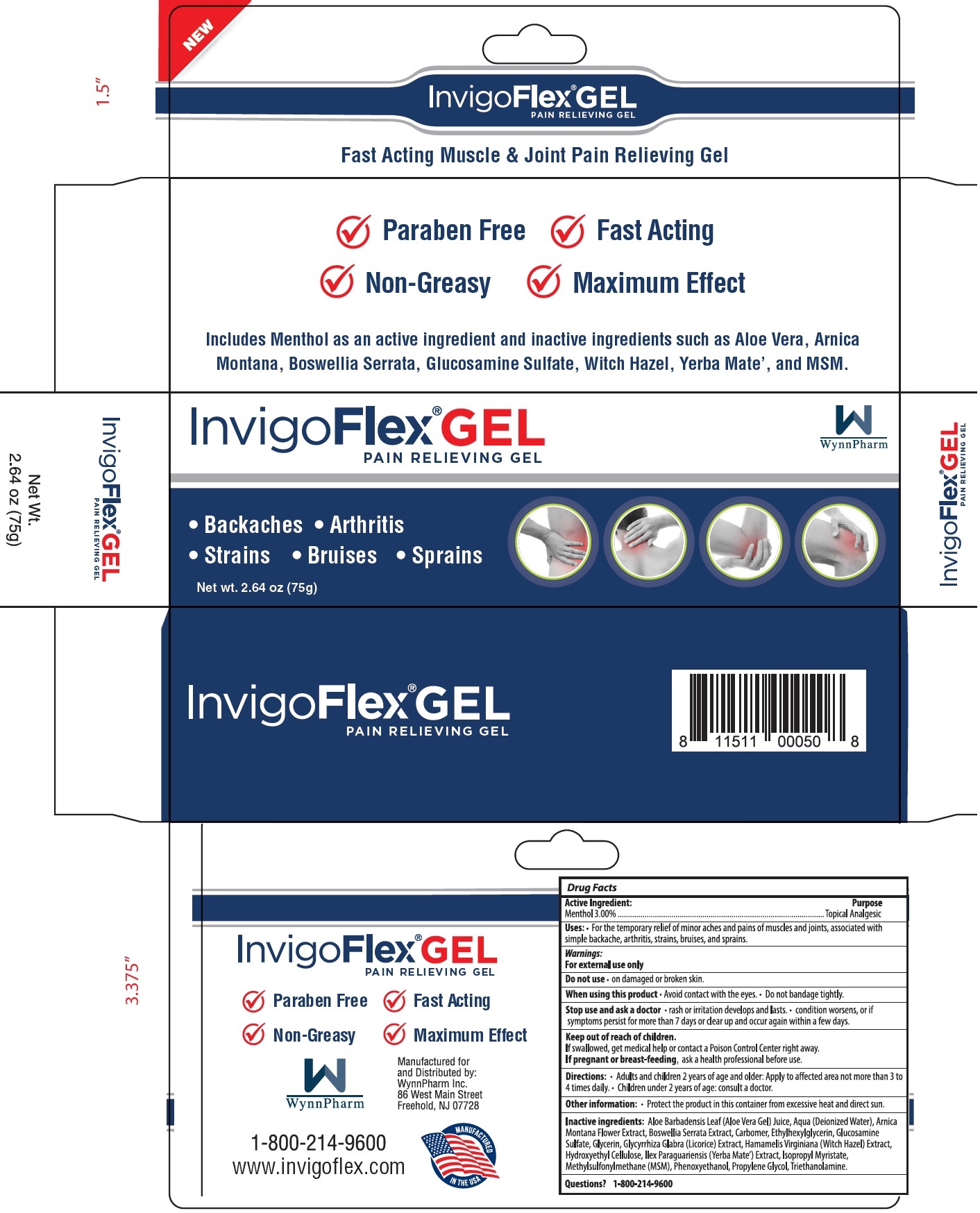
Label: INVIGO FLEX PAIN RELIEF GEL- menthol gel
- NDC Code(s): 35324-276-00
- Packager: WYNNPHARM INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient:
- Uses:
-
Warnings:
For external use only
Stop use and ask a doctor
• rash or irritation develops and lasts. • condition worsens, or if symptoms persist for more than 7 days or clears up and occur again within a few days.
- Directions
- Other information
-
Inactive Ingredients:
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Arnica Montana Flower Extract, Boswellia Serrata Extract, Carbomer, Ethylhexylglycerin, Glucosamine Sulfate, Glycerin, GLycyrrhiza Glabra (Licorice) Extract, Hamamelis Virginiana (Witch Hazel) Extract, Hydroxyethyl Cellulose, Ilex Paraguariensis (Yerba Mate') Extract, Isopropyl Myristate, Methylsulfonulmethane (MSM), Phenoxyethanol, Propylene Glycol, Triethanolamine.
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
INVIGO FLEX PAIN RELIEF GEL
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:35324-276 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 30 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) GLYCERIN (UNII: PDC6A3C0OX) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35324-276-00 1 in 1 BOX 02/15/2022 1 75 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/15/2022 Labeler - WYNNPHARM INC. (620885173)