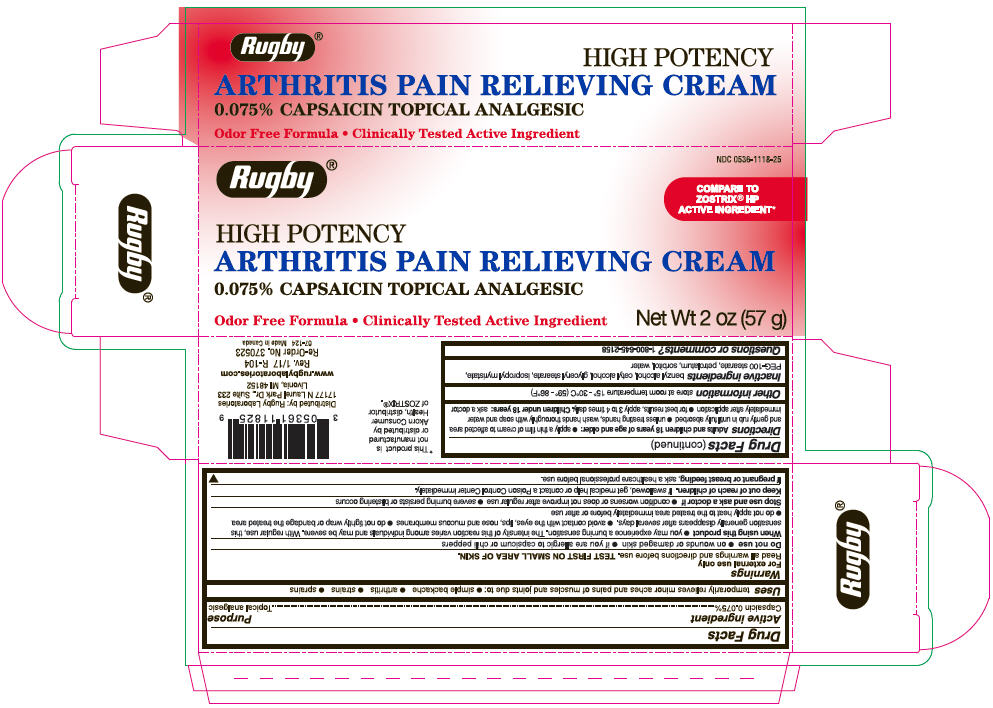
Label: RUGBY ARTHRITIS PAIN RELIEVING TOPICAL ANALGESIC- capsaicin cream
- NDC Code(s): 0536-1118-25
- Packager: Rugby Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Read all warnings and directions before use. TEST FIRST ON SMALL AREA OF SKIN.
When using this product
- ♦
- you may experience a burning sensation. The intensity of this reaction varies among individuals and may be severe. With regular use, this sensation generally disappears after several days.
- ♦
- avoid contact with eyes, lips, nose and mucous membranes
- ♦
- do not tightly wrap or bandage the treated area
- ♦
- do not apply heat to the treated area immediately before or after use
Stop and ask a doctor if
- ♦
- condition worsens or does not improve after regular use
- ♦
- severe burning persists or blistering occurs
-
Directions
Adults and children 18 years of age and older:
- ♦
- apply a thin film of cream to affected area and gently rub in until fully absorbed
- ♦
- unless treating hands, wash hands thoroughly with soap and water immediately after application
- ♦
- for best results, apply 3 to 4 times daily.
Children under 18 years: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 57 g Tube Carton
-
INGREDIENTS AND APPEARANCE
RUGBY ARTHRITIS PAIN RELIEVING TOPICAL ANALGESIC
capsaicin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Capsaicin (UNII: S07O44R1ZM) (Capsaicin - UNII:S07O44R1ZM) Capsaicin 0.75 mg in 1 g Inactive Ingredients Ingredient Name Strength Benzyl Alcohol (UNII: LKG8494WBH) Cetyl Alcohol (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) Isopropyl Myristate (UNII: 0RE8K4LNJS) PEG-100 Stearate (UNII: YD01N1999R) Petrolatum (UNII: 4T6H12BN9U) Sorbitol (UNII: 506T60A25R) Water (UNII: 059QF0KO0R) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1118-25 1 in 1 CARTON 05/24/2017 1 57 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 05/24/2017 Labeler - Rugby Laboratories (079246066) Registrant - Garcoa, Inc (036464697) Establishment Name Address ID/FEI Business Operations Garcoa, Inc 036464697 MANUFACTURE(0536-1118)